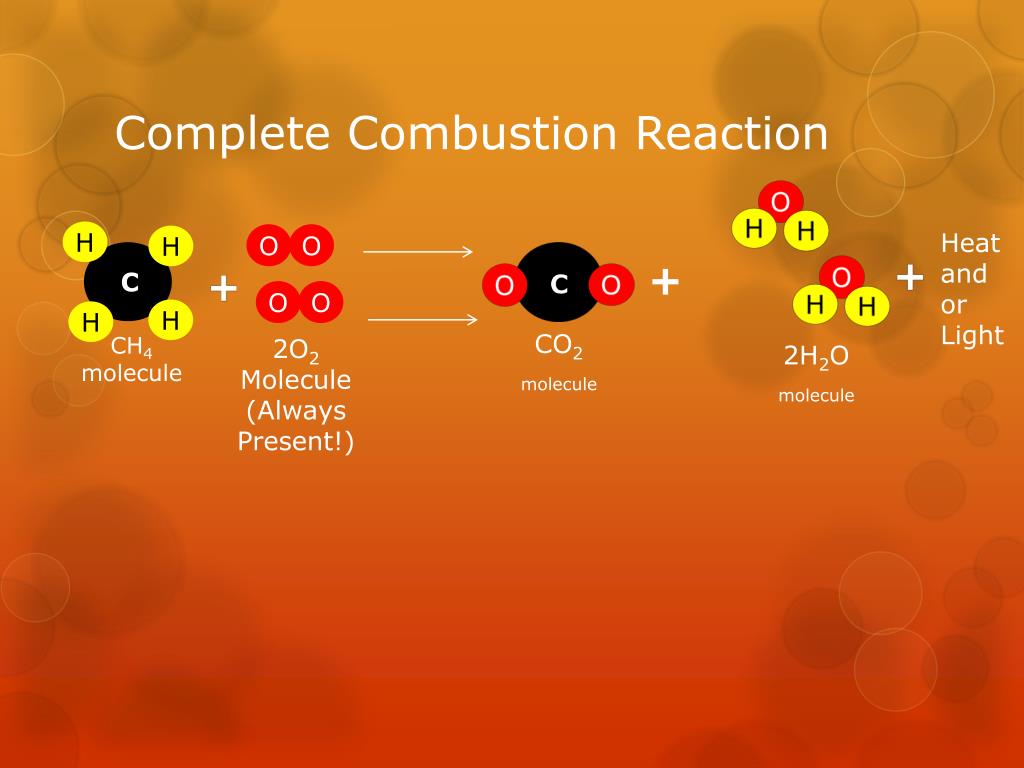
Chemistry Definition Of Combustion . Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
from www.slideserve.com
Heat provides the activation energy to start the chemical reaction. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat.
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID
Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat.
From www.slideshare.net
Heat of combustion of Various Alcohols Chemistry Definition Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion. Chemistry Definition Of Combustion.
From www.abcworksheet.com
What is Combustion Definition of Combustion Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a type of chemical. Chemistry Definition Of Combustion.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc. Chemistry Definition Of Combustion.
From www.britannica.com
Combustion Definition, Reaction, Analysis, & Facts Britannica Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Learn the definition of combustion, the equation for combustion and. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas,. Chemistry Definition Of Combustion.
From www.youtube.com
Definition of Combustion for class 8 science. YouTube Chemistry Definition Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal,. Chemistry Definition Of Combustion.
From sciencenotes.org
Combustion Reaction Definition and Examples Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. Combustion is a reaction between a hydrocarbon fuel. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Chemistry Definition Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT 5 Types of Chemical Reactions PowerPoint Presentation, free Chemistry Definition Of Combustion Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o),. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT How Can We Describe Chemical Reactions? PowerPoint Presentation Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form. Chemistry Definition Of Combustion.
From www.slideshare.net
Combustion Reactions Slideshow123 Chemistry Definition Of Combustion Heat provides the activation energy to start the chemical reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a. Chemistry Definition Of Combustion.
From slideserve.com
PPT Chapter 5 Chemical Reactions and Quantities PowerPoint Chemistry Definition Of Combustion Heat provides the activation energy to start the chemical reaction. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Chemistry Definition Of Combustion.
From www.slideshare.net
Classification of Chemical Reactions Chemistry Definition Of Combustion Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical. Chemistry Definition Of Combustion.
From www.youtube.com
Balancing Combustion Reactions Chemistry Tutorial YouTube Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for. Chemistry Definition Of Combustion.
From treatybottle13.pythonanywhere.com
Simple Combustion Reaction Octane Physics Formulas Of Class 12 Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for. Chemistry Definition Of Combustion.
From studylib.net
3. Combustion Chemistry Chemistry Definition Of Combustion Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide. Chemistry Definition Of Combustion.
From gamesmartz.com
Combustion Definition & Image GameSmartz Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas,. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. A combustion. Chemistry Definition Of Combustion.
From primarybda.weebly.com
4 types of combustion primarybda Chemistry Definition Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3. Chemistry Definition Of Combustion.
From sciencetrends.com
Combustion Reaction Examples And Definition Science Trends Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the. Chemistry Definition Of Combustion.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. Combustion, a. Chemistry Definition Of Combustion.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for. Chemistry Definition Of Combustion.
From www.youtube.com
Balancing Combustion Reactions YouTube Chemistry Definition Of Combustion Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID Chemistry Definition Of Combustion Heat provides the activation energy to start the chemical reaction. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is. Chemistry Definition Of Combustion.
From www.thoughtco.com
Combustion Definition in Chemistry Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction. Chemistry Definition Of Combustion.
From www.teachoo.com
Different Types of Combustion with Examples Teachoo Concepts Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Learn. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT CHAPTER 6 COMBUSTION AND FLAME PowerPoint Presentation, free Chemistry Definition Of Combustion Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light. Chemistry Definition Of Combustion.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon. Chemistry Definition Of Combustion.
From www.pinterest.co.kr
Combustion Reaction Definition, Characteristics & Examples Carbon Chemistry Definition Of Combustion Heat provides the activation energy to start the chemical reaction. A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including. Chemistry Definition Of Combustion.
From slideshare.net
form1sciencechapter5part2 Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of. Chemistry Definition Of Combustion.
From www.shmoop.com
Combustion Shmoop Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry. Chemistry Definition Of Combustion.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube Chemistry Definition Of Combustion A combustion reaction is a type of chemical reaction in which a compound and an oxidant are reacted to. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the. Chemistry Definition Of Combustion.
From www.youtube.com
Examples of Combustion Reactions YouTube Chemistry Definition Of Combustion Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Learn the definition of combustion, the equation for. Chemistry Definition Of Combustion.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Chemistry Definition Of Combustion A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat. A combustion reaction is a type of chemical reaction in. Chemistry Definition Of Combustion.
From www.showme.com
Combustion Reaction Science, Chemistry, Chemicalreactions ShowMe Chemistry Definition Of Combustion Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a. Chemistry Definition Of Combustion.