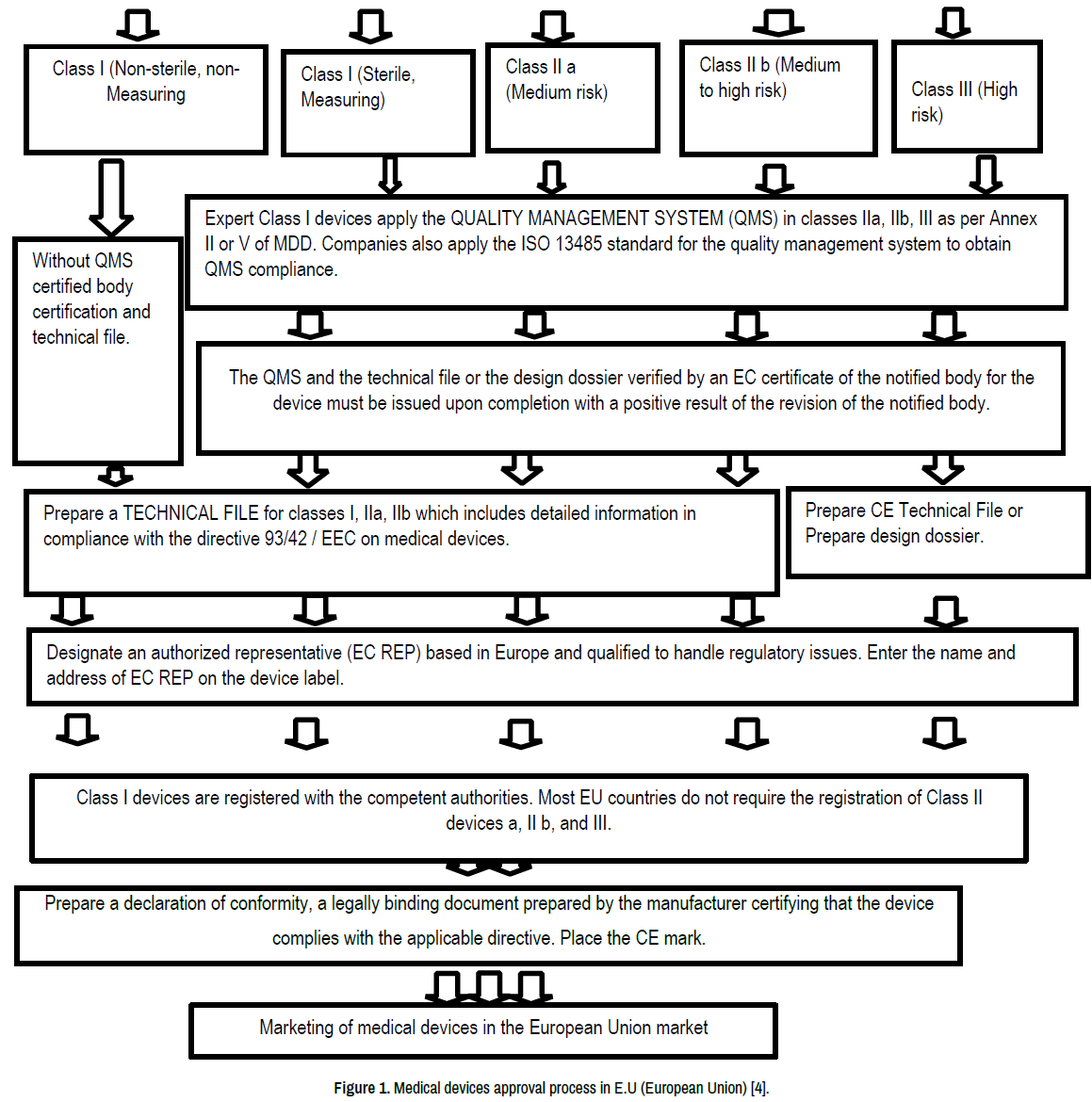
Medical Device Regulations Wikipedia . Medical device regulation act of 1976 in the united. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european union. Strengthen transparency of information related to medical devices for consumers and. Improve the quality, safety and reliability of medical devices placed on the european market. Medical device regulation may refer to: Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the.
from www.hilarispublisher.com
Strengthen transparency of information related to medical devices for consumers and. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 in the european union. In the european union (eu) they must undergo a conformity. Improve the quality, safety and reliability of medical devices placed on the european market. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Medical device regulation act of 1976 in the united. Medical device regulation may refer to:
pharmaceuticalregulatoryaffairsprocess
Medical Device Regulations Wikipedia In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Medical device regulation may refer to: In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Improve the quality, safety and reliability of medical devices placed on the european market. Strengthen transparency of information related to medical devices for consumers and. Medical device regulation act of 1976 in the united. Regulation (eu) 2017/745 in the european union. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the.
From blog.cosmotrace.com
Medical Devices Regulations (MDR) Medical Device Regulations Wikipedia Strengthen transparency of information related to medical devices for consumers and. Medical devices are products or equipment intended for a medical purpose. Medical device regulation act of 1976 in the united. In the european union (eu) they must undergo a conformity. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing. Medical Device Regulations Wikipedia.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV Medical Device Regulations Wikipedia The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 in the european union. Medical device regulation act of 1976 in the united. Regulation (eu) 2017/745 of. Medical Device Regulations Wikipedia.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Regulations Wikipedia In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. Improve the quality, safety and reliability of medical devices placed. Medical Device Regulations Wikipedia.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Wikipedia Medical device regulation may refer to: In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical devices are products or. Medical Device Regulations Wikipedia.
From www.apcerls.com
US Medical Device Regulations APCER Life Sciences Medical Device Regulations Wikipedia Medical device regulation act of 1976 in the united. Strengthen transparency of information related to medical devices for consumers and. Regulation (eu) 2017/745 in the european union. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Improve the quality, safety and reliability of medical devices placed on the. Medical Device Regulations Wikipedia.
From www.eclevarmedtech.com
A Guide to Medical Devices Regulations Everything You Need to Know Medical Device Regulations Wikipedia Medical devices are products or equipment intended for a medical purpose. Medical device regulation act of 1976 in the united. Regulation (eu) 2017/745 in the european union. Improve the quality, safety and reliability of medical devices placed on the european market. In the european union (eu) they must undergo a conformity. Strengthen transparency of information related to medical devices for. Medical Device Regulations Wikipedia.
From www.scribd.com
Medical device Regulations PDF Medical Device Verification And Medical Device Regulations Wikipedia Strengthen transparency of information related to medical devices for consumers and. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 in the european union. The eu commission published updated versions of its factsheet for manufacturers. Medical Device Regulations Wikipedia.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Regulations Wikipedia Medical device regulation may refer to: The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical devices are products or equipment intended for a medical purpose. Strengthen transparency of information related to medical devices for consumers and. In the european union (eu) they must undergo a conformity. Improve the quality,. Medical Device Regulations Wikipedia.
From www.youtube.com
Understanding Medical Device Regulations YouTube Medical Device Regulations Wikipedia In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european union. Medical device regulation act of 1976 in the united. In the european union (eu) they must undergo a conformity. Strengthen transparency of information related to medical devices for consumers and. The eu commission. Medical Device Regulations Wikipedia.
From www.presentationeze.com
Global Medical Device Regulation PresentationEZE Medical Device Regulations Wikipedia Regulation (eu) 2017/745 in the european union. Medical devices are products or equipment intended for a medical purpose. Strengthen transparency of information related to medical devices for consumers and. Medical device regulation may refer to: Medical device regulation act of 1976 in the united. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Medical Device Regulations Wikipedia.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Medical Device Regulations Wikipedia Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 in the european union. Strengthen transparency of information related to medical devices for consumers and. In the european union (eu) they must undergo a conformity. Medical device regulation may refer to: Medical devices are products or equipment. Medical Device Regulations Wikipedia.
From www.tuvsud.com
Infographic The New Medical Device Regulation TÜV SÜD Medical Device Regulations Wikipedia Strengthen transparency of information related to medical devices for consumers and. Medical device regulation act of 1976 in the united. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 in the european union. Regulation (eu) 2017/745. Medical Device Regulations Wikipedia.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Wikipedia Improve the quality, safety and reliability of medical devices placed on the european market. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 in the european union. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. In the 1960s and 1970s, congress responded to the public’s. Medical Device Regulations Wikipedia.
From www.cell.com
The Regulation of Wearable Medical Devices Trends in Biotechnology Medical Device Regulations Wikipedia Improve the quality, safety and reliability of medical devices placed on the european market. Medical device regulation may refer to: In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 in the european union. The eu commission. Medical Device Regulations Wikipedia.
From www.apcerls.com
US Medical Device Regulations APCER Life Sciences Medical Device Regulations Wikipedia The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Improve the quality, safety and reliability of medical devices placed on the european market. In the european union (eu) they must. Medical Device Regulations Wikipedia.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulations Wikipedia Medical device regulation may refer to: In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european union. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended. Medical Device Regulations Wikipedia.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Regulations Wikipedia The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 in the european union. Medical device regulation may refer to: Improve the quality, safety and reliability of medical devices placed on the european market. Medical device regulation act. Medical Device Regulations Wikipedia.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulations Wikipedia Medical devices are products or equipment intended for a medical purpose. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical device regulation may refer to: Improve the quality, safety and reliability of medical devices placed on the european market. Strengthen transparency of information related to medical devices for consumers. Medical Device Regulations Wikipedia.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Regulations Wikipedia Strengthen transparency of information related to medical devices for consumers and. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical device regulation act of 1976 in the united. In the 1960s and 1970s, congress responded to the public’s. Medical Device Regulations Wikipedia.
From www.vchri.ca
Medical Device Regulations and Guidelines VCH Research Institute Medical Device Regulations Wikipedia Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Medical device regulation may refer to: Improve the quality, safety and reliability of medical devices placed on the european. Medical Device Regulations Wikipedia.
From www.castoredc.com
Overview of EU Medical Device Regulations (MDR) Medical Device Regulations Wikipedia Improve the quality, safety and reliability of medical devices placed on the european market. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european. Medical Device Regulations Wikipedia.
From www.slideshare.net
Medical Device Regulation Medical Device Regulations Wikipedia In the european union (eu) they must undergo a conformity. Strengthen transparency of information related to medical devices for consumers and. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european union. Medical device regulation act of 1976 in the united. The eu commission. Medical Device Regulations Wikipedia.
From www.apcerls.com
EU Medical Device Regulations APCER Life Sciences Medical Device Regulations Wikipedia Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical device regulation act of 1976 in the united. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. In the 1960s and 1970s, congress responded to the public’s desire for. Medical Device Regulations Wikipedia.
From crfweb.com
Medical Device Regulations Medical Device Regulations Wikipedia Medical device regulation act of 1976 in the united. Regulation (eu) 2017/745 in the european union. Improve the quality, safety and reliability of medical devices placed on the european market. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. In the european union (eu) they must undergo a conformity. Medical. Medical Device Regulations Wikipedia.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Regulations Wikipedia Medical devices are products or equipment intended for a medical purpose. Medical device regulation act of 1976 in the united. In the european union (eu) they must undergo a conformity. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 of the european parliament and of the. Medical Device Regulations Wikipedia.
From www.ignitec.com
UK medical device regulations glossary What every medical... Medical Device Regulations Wikipedia Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. Medical device regulation may refer to: Improve the quality, safety and reliability of medical devices placed on the european. Medical Device Regulations Wikipedia.
From www.studypool.com
SOLUTION Medical device regulations global overview and guiding Medical Device Regulations Wikipedia Medical device regulation may refer to: Regulation (eu) 2017/745 in the european union. Strengthen transparency of information related to medical devices for consumers and. Improve the quality, safety and reliability of medical devices placed on the european market. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the. Medical Device Regulations Wikipedia.
From www.slideserve.com
PPT Global Medical Device Regulations PowerPoint Presentation, free Medical Device Regulations Wikipedia Medical device regulation act of 1976 in the united. In the european union (eu) they must undergo a conformity. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Regulation (eu) 2017/745 in the european union. Medical device regulation may refer to: Strengthen transparency of information related to medical devices for. Medical Device Regulations Wikipedia.
From www.hilarispublisher.com
pharmaceuticalregulatoryaffairsprocess Medical Device Regulations Wikipedia Regulation (eu) 2017/745 in the european union. In the european union (eu) they must undergo a conformity. Medical device regulation act of 1976 in the united. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Improve the quality, safety and reliability of medical devices placed on the european market. Medical. Medical Device Regulations Wikipedia.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Wikipedia The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Medical device regulation act of 1976 in the united. Strengthen transparency of information related to medical devices for consumers and. Regulation (eu) 2017/745 in the european union. Medical devices are products or equipment intended for a medical purpose. In the 1960s. Medical Device Regulations Wikipedia.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulations Wikipedia Strengthen transparency of information related to medical devices for consumers and. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Regulation (eu) 2017/745 in the european union. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The. Medical Device Regulations Wikipedia.
From info.dicksondata.com
INFOGRAPHIC History of Medical Device Regulation Medical Device Regulations Wikipedia Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Medical device regulation may refer to: Regulation (eu) 2017/745 in the european union. Improve the quality, safety and reliability. Medical Device Regulations Wikipedia.
From www.slideshare.net
Global medical device regulations Medical Device Regulations Wikipedia Improve the quality, safety and reliability of medical devices placed on the european market. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Medical devices are products or equipment. Medical Device Regulations Wikipedia.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Regulations Wikipedia Regulation (eu) 2017/745 in the european union. In the european union (eu) they must undergo a conformity. Strengthen transparency of information related to medical devices for consumers and. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Regulation (eu) 2017/745 of the european parliament and of the council of 5. Medical Device Regulations Wikipedia.
From www.stendard.io
6 Major Implementations in the EU Medical Devices Regulation (MDR Medical Device Regulations Wikipedia In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The eu commission published updated versions of its factsheet for manufacturers of medical devices and implementation model for the. Strengthen transparency of information related to medical devices for consumers and. Medical devices are products or equipment intended for a medical. Medical Device Regulations Wikipedia.