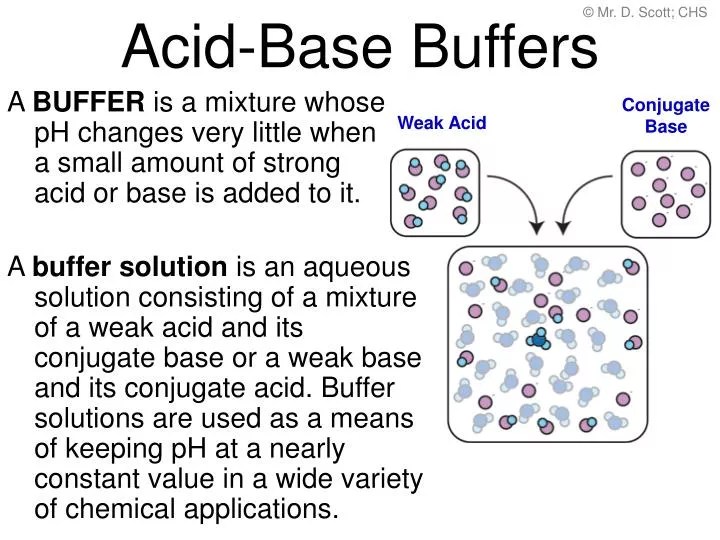
What Is A Buffer Acid Base . A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from www.slideserve.com
A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487
What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.
From www.youtube.com
26 Acid Base Buffers YouTube What Is A Buffer Acid Base A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. It is able to neutralize. What Is A Buffer Acid Base.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. What Is A Buffer Acid Base.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Is A Buffer Acid Base A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or base, thus. Buffer. What Is A Buffer Acid Base.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online presentation What Is A Buffer Acid Base A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It consists of a solution of a weak acid and its conjugate base, or vice versa. Buffer solutions resist a change in ph when small. In chemistry, the definition of a buffer is. What Is A Buffer Acid Base.
From www.studypool.com
SOLUTION Biochemistry ph buffer acid base and their role in body Studypool What Is A Buffer Acid Base A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. It is able to neutralize. What Is A Buffer Acid Base.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called. What Is A Buffer Acid Base.
From www.youtube.com
Buffer from weak acid and strong base YouTube What Is A Buffer Acid Base A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change in ph when small. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a. What Is A Buffer Acid Base.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a. What Is A Buffer Acid Base.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a. What Is A Buffer Acid Base.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology YouTube What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change in ph when small.. What Is A Buffer Acid Base.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Buffer Acid Base A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It consists of a solution of a weak acid and its conjugate base, or vice versa. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist. What Is A Buffer Acid Base.
From lagccnsdoer.commons.gc.cuny.edu
SCB 115 Lab 2 Exercise 4 pH, Acids, Bases, and Buffers Natural Sciences Open Educational What Is A Buffer Acid Base Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution. What Is A Buffer Acid Base.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Types of Buffer What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation, free download ID257615 What Is A Buffer Acid Base Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture of a weak. What Is A Buffer Acid Base.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID3141916 What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It consists of a solution of a weak acid and its. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Atoms, Molecules, Ions, Acids, Bases, Buffers, pH PowerPoint Presentation ID5847414 What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the. What Is A Buffer Acid Base.
From ditki.com
Physiology Glossary Acids & Bases ditki medical & biological sciences What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. It consists of a. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph when small. It is able to neutralize small amounts of added acid or. What Is A Buffer Acid Base.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Alkalosis What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Unit 3 Acids and Bases PowerPoint Presentation, free download ID2090128 What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a. What Is A Buffer Acid Base.
From www.studypool.com
SOLUTION Biochemistry ph buffer acid base and their role in body Studypool What Is A Buffer Acid Base A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions resist a change in ph when small. It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture. What Is A Buffer Acid Base.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid Base A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of. What Is A Buffer Acid Base.
From acidsandbasesfordummieschem.weebly.com
Buffers Acid and Bases for Dummies! What Is A Buffer Acid Base A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change in ph when small. In chemistry, the definition of. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Presentation ID3301970 What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A. What Is A Buffer Acid Base.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its conjugate base, or vice versa. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of. What Is A Buffer Acid Base.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A mixture of a. What Is A Buffer Acid Base.
From www.youtube.com
Acid, Base, and Buffer Chemistry YouTube What Is A Buffer Acid Base A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. What Is A Buffer Acid Base.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Presentation ID3301970 What Is A Buffer Acid Base It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of. What Is A Buffer Acid Base.
From courses.lumenlearning.com
Buffers Chemistry What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. Buffer solutions resist a change in ph when small. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or. What Is A Buffer Acid Base.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition. What Is A Buffer Acid Base.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is A Buffer Acid Base In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A mixture of a. What Is A Buffer Acid Base.
From fyognlubk.blob.core.windows.net
What Is A Buffer In Biology at Earl Lovelace blog What Is A Buffer Acid Base A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions resist a change in ph when small. In chemistry, the definition of a buffer is a solution. What Is A Buffer Acid Base.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID556590 What Is A Buffer Acid Base It consists of a solution of a weak acid and its conjugate base, or vice versa. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the. What Is A Buffer Acid Base.