Magnesium Sulfate In Water Equation . When acids react with a base, a salt and water are made. Acid + base → salt + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Nitric acid + magnesium oxide → magnesium nitrate +. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Three moles of solid magnesium [mg] and four moles of. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Determine the degree of hydration and write the formula of hydrated.
from www.numerade.com
It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Nitric acid + magnesium oxide → magnesium nitrate +. Three moles of solid magnesium [mg] and four moles of. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. When acids react with a base, a salt and water are made. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Acid + base → salt + water. Determine the degree of hydration and write the formula of hydrated.
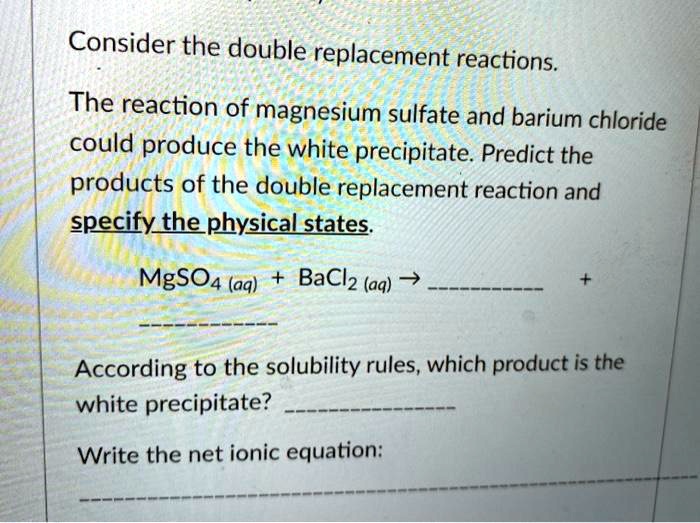
SOLVED Consider the double replacement reactions. The reaction of
Magnesium Sulfate In Water Equation Hydrated magnesium sulfate contains 51.2 % water of crystallisation. When acids react with a base, a salt and water are made. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Determine the degree of hydration and write the formula of hydrated. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Nitric acid + magnesium oxide → magnesium nitrate +. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Acid + base → salt + water. Three moles of solid magnesium [mg] and four moles of.
From www.youtube.com
Equation for MgSO4 + H2O (Magnesium sulfate + Water) YouTube Magnesium Sulfate In Water Equation When acids react with a base, a salt and water are made. Nitric acid + magnesium oxide → magnesium nitrate +. Three moles of solid magnesium [mg] and four moles of. Acid + base → salt + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content. Magnesium Sulfate In Water Equation.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Magnesium Sulfate In Water Equation The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Three moles of solid magnesium [mg] and four moles of. When acids react with a base, a salt and water are made. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double.. Magnesium Sulfate In Water Equation.
From www.slideserve.com
PPT Hydrates PowerPoint Presentation, free download ID2110313 Magnesium Sulfate In Water Equation Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Mg(oh)2 + h2so4. Magnesium Sulfate In Water Equation.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Magnesium Sulfate In Water Equation Nitric acid + magnesium oxide → magnesium nitrate +. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. When acids react with a base, a salt and water are made. Determine the degree of hydration and write the formula of hydrated. It can also be noted that the. Magnesium Sulfate In Water Equation.
From stock.adobe.com
Molecular formula and chemical structure of magnesium sulfate vector de Magnesium Sulfate In Water Equation Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Acid + base → salt + water. Nitric acid + magnesium oxide → magnesium nitrate +. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Three moles of solid magnesium [mg] and four moles of. Determine the degree of. Magnesium Sulfate In Water Equation.
From mcscience.co.uk
Chemical Reactions McScience KS3 Magnesium Sulfate In Water Equation When acids react with a base, a salt and water are made. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Determine the degree of hydration and write the formula of hydrated. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Mg(oh)2 + h2so4 = mg(so4) + h2o is a. Magnesium Sulfate In Water Equation.
From www.alamy.com
Magnesium sulfate, chemical structure, illustration Stock Photo Alamy Magnesium Sulfate In Water Equation It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Mg(oh)2 + h2so4 =. Magnesium Sulfate In Water Equation.
From www.numerade.com
SOLVED Consider the double replacement reactions. The reaction of Magnesium Sulfate In Water Equation Three moles of solid magnesium [mg] and four moles of. Nitric acid + magnesium oxide → magnesium nitrate +. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Determine the degree of hydration and write the formula of hydrated. The magnesium sulfate ion pair complex (mgso4) is the most significant. Magnesium Sulfate In Water Equation.
From www.researchgate.net
Magnesium sulfate solubility at various temperatures. Download Magnesium Sulfate In Water Equation Acid + base → salt + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Determine the degree of hydration and write the formula of hydrated. Magnesium hydroxide + sulfuric acid. Magnesium Sulfate In Water Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate heptahydrate YouTube Magnesium Sulfate In Water Equation Determine the degree of hydration and write the formula of hydrated. Three moles of solid magnesium [mg] and four moles of. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. The. Magnesium Sulfate In Water Equation.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Magnesium Sulfate In Water Equation The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Acid + base. Magnesium Sulfate In Water Equation.
From www.numerade.com
SOLVEDAn aqueous stream contains 5 by weight of magnesium sulfate Magnesium Sulfate In Water Equation Hydrated magnesium sulfate contains 51.2 % water of crystallisation. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Three moles of solid magnesium [mg] and four moles of. It can also be noted that the solubility of magnesium sulfate in water. Magnesium Sulfate In Water Equation.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Magnesium Sulfate In Water Equation Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Determine the degree of hydration and write the formula of hydrated. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Acid + base → salt + water. Three moles of solid magnesium [mg] and four moles of. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Nitric. Magnesium Sulfate In Water Equation.
From www.researchgate.net
(PDF) Conductivity of Magnesium Sulfate in Water from 5 to 35°C and Magnesium Sulfate In Water Equation Acid + base → salt + water. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Determine the degree of hydration and write the formula of hydrated. When acids react with a base, a salt and water are made.. Magnesium Sulfate In Water Equation.
From askfilo.com
Question 32 g of hydrated magnesium sulphate MgSO4 ⋅XH2 O is dissolved in.. Magnesium Sulfate In Water Equation When acids react with a base, a salt and water are made. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Acid + base → salt + water. Three moles of solid magnesium [mg] and four moles of. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium. Magnesium Sulfate In Water Equation.
From www.toppr.com
Molecular formula of Glauber salt is Magnesium Sulfate In Water Equation Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Acid + base → salt + water. Determine the degree of. Magnesium Sulfate In Water Equation.
From www.slideserve.com
PPT Hydrates PowerPoint Presentation, free download ID2110313 Magnesium Sulfate In Water Equation The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Nitric acid + magnesium oxide → magnesium nitrate +. Acid + base → salt + water. Mg(oh)2 + h2so4 = mg(so4). Magnesium Sulfate In Water Equation.
From www.youtube.com
H2SO4+Mg(OH)2=H2O+MgSO4 Balanced EquationSulphuric Acid+Magnesium Magnesium Sulfate In Water Equation Three moles of solid magnesium [mg] and four moles of. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Determine the degree of hydration and write the formula of hydrated. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. When acids react with a base, a salt and water are made. Nitric acid + magnesium oxide → magnesium nitrate. Magnesium Sulfate In Water Equation.
From www.toppr.com
Convert the following word equations into molecular equation and Magnesium Sulfate In Water Equation Hydrated magnesium sulfate contains 51.2 % water of crystallisation. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Three moles of solid magnesium [mg] and four moles of. Acid + base → salt + water. Determine the degree of hydration and write the formula of hydrated. Magnesium hydroxide + sulfuric. Magnesium Sulfate In Water Equation.
From testbook.com
Magnesium Sulphate Formula Know Structure, Properties and Uses Magnesium Sulfate In Water Equation Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. When acids react with a base, a salt and water are made. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively.. Magnesium Sulfate In Water Equation.
From www.chegg.com
Solved Write the formula equation from the word equation. Magnesium Sulfate In Water Equation Three moles of solid magnesium [mg] and four moles of. Nitric acid + magnesium oxide → magnesium nitrate +. Acid + base → salt + water. Determine the degree of hydration and write the formula of hydrated. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in. Magnesium Sulfate In Water Equation.
From www.nagwa.com
Question Video Determining the Water of Hydration of Magnesium Sulfate Magnesium Sulfate In Water Equation Nitric acid + magnesium oxide → magnesium nitrate +. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Acid + base → salt + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh. Magnesium Sulfate In Water Equation.
From animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Sulfate In Water Equation Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Nitric acid + magnesium oxide → magnesium nitrate +. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Acid + base → salt + water. Determine the degree of hydration and write the formula of hydrated. It can also be noted that the solubility of magnesium sulfate in water increases. Magnesium Sulfate In Water Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Magnesium Sulfate In Water Equation Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Acid + base → salt + water. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Determine the degree of hydration and write the formula of hydrated. Nitric acid + magnesium oxide → magnesium nitrate +. The magnesium sulfate ion pair complex (mgso4) is the most significant complex. Magnesium Sulfate In Water Equation.
From bons-up.blogspot.com
Magnesium Reaction With Water / Hydrochloric acid Magnificent Magnesium Sulfate In Water Equation Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Nitric acid + magnesium oxide → magnesium nitrate +. When acids react with a base, a salt and water are made. Determine the degree of hydration and write the formula of hydrated. Three moles of solid magnesium [mg] and. Magnesium Sulfate In Water Equation.
From www.numerade.com
SOLVED Magnesium sulfate can exist as an anhydrous solid salt or it Magnesium Sulfate In Water Equation Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Nitric acid + magnesium oxide → magnesium nitrate +. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Determine the degree of hydration and write the formula of hydrated. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double.. Magnesium Sulfate In Water Equation.
From segudanggambar753.blogspot.com
Magnesium Sulfate Heptahydrate Hydrate Formula Magnesium Sulfate Magnesium Sulfate In Water Equation Hydrated magnesium sulfate contains 51.2 % water of crystallisation. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. When acids react with a base, a salt and water are made. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium. Magnesium Sulfate In Water Equation.
From www.scuola-e-cultura.it
Solfato di magnesio Formula Uso come lassativo Scuola e cultura Magnesium Sulfate In Water Equation Three moles of solid magnesium [mg] and four moles of. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. When acids react with a base, a salt and water. Magnesium Sulfate In Water Equation.
From www.numerade.com
SOLVEDIf 1.00 g of magnesium hydroxide reacts with 0.605 g of sulfuric Magnesium Sulfate In Water Equation Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Nitric acid + magnesium oxide → magnesium nitrate +. When acids react with a base, a salt and water are made.. Magnesium Sulfate In Water Equation.
From biobasic-asia.com
Magnesium Sulfate, anhydrous Bio Basic Asia Pacific Pte Ltd Magnesium Sulfate In Water Equation When acids react with a base, a salt and water are made. Nitric acid + magnesium oxide → magnesium nitrate +. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. The magnesium sulfate ion pair complex (mgso4) is the most significant. Magnesium Sulfate In Water Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate YouTube Magnesium Sulfate In Water Equation Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. When acids react with a base, a salt and water are made. Determine the degree of hydration and write the formula. Magnesium Sulfate In Water Equation.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium Sulfate In Water Equation Nitric acid + magnesium oxide → magnesium nitrate +. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. Acid + base → salt + water. The magnesium sulfate ion pair complex (mgso4) is the most significant complex present, representing 2.6% and 11% of the total magnesium content in fresh and sea water, respectively. It can also be noted that the. Magnesium Sulfate In Water Equation.
From www.shalom-education.com
Testing for Sulfate Ions GCSE Chemistry Revision Magnesium Sulfate In Water Equation It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. Nitric acid + magnesium oxide → magnesium nitrate +. Acid + base → salt + water. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Three moles of solid magnesium. Magnesium Sulfate In Water Equation.
From www.toppr.com
The equation shows the reaction between magnesium and sulphuric acid Magnesium Sulfate In Water Equation Nitric acid + magnesium oxide → magnesium nitrate +. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. Magnesium + sulfuric acid = magnesium sulfate + sulfur + water. Three moles of solid magnesium [mg] and four moles of. Hydrated magnesium sulfate contains 51.2 % water of crystallisation. It can also be noted that the solubility of magnesium sulfate. Magnesium Sulfate In Water Equation.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Sulfate In Water Equation Three moles of solid magnesium [mg] and four moles of. Magnesium hydroxide + sulfuric acid = magnesium sulfate + water. When acids react with a base, a salt and water are made. Mg(oh)2 + h2so4 = mg(so4) + h2o is a double. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is. Magnesium Sulfate In Water Equation.