Ground Term Symbol For D5 . The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The ground state term symbol for d5 is 6s. Please explain how to get the answer with the necessary. Write the term symbol for the ground state. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0.
from www.chegg.com
Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Please explain how to get the answer with the necessary. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). The ground state term symbol for d5 is 6s. Write the term symbol for the ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state.
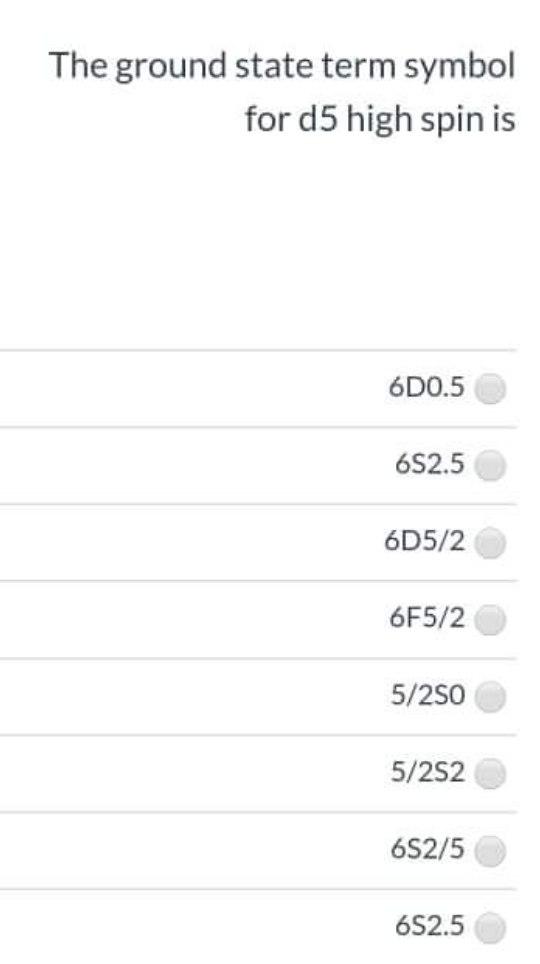
Solved The ground state term symbol for d5 high spin is
Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). The ground state term symbol for d5 is 6s. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Please explain how to get the answer with the necessary. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy.
From www.vrogue.co
Solveddetermine The Ground State Term Symbol Of The A vrogue.co Ground Term Symbol For D5 Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular. Ground Term Symbol For D5.
From edurev.in
The ground state term symbols for high spin d5s1 and d5 configurations, respectively area)3S Ground Term Symbol For D5 Write the term symbol for the ground state. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Please explain how to get the answer with the necessary. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The answer. Ground Term Symbol For D5.
From www.slideserve.com
PPT NSRRC September 12 program PowerPoint Presentation, free download ID6606637 Ground Term Symbol For D5 Please explain how to get the answer with the necessary. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of. Ground Term Symbol For D5.
From stock.adobe.com
Three different grounds symbols. Electrical symbols. Protective earth ground symbol. Earth Ground Term Symbol For D5 The ground state term symbol for d5 is 6s. Write the term symbol for the ground state. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. This is because. Ground Term Symbol For D5.
From www.numerade.com
What is the ground state term symbol for each of the following dn configurations 1. d2 ii. ds Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The ground state term symbol for d5 is 6s. Please explain how to get the answer with the necessary. The answer to. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Write the term symbol for the ground state. This is because the five electrons in the d orbital can have. Ground Term Symbol For D5.
From www.youtube.com
Ground state term symbol or term symbol for lowest energy level chemistry Ground Term Symbol For D5 The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The ground state. Ground Term Symbol For D5.
From www.chegg.com
Solved The ground state term symbol for d5 high spin is Ground Term Symbol For D5 Please explain how to get the answer with the necessary. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The ground state term symbol for d5 is 6s. Write the term symbol for the ground state. This is because the five electrons in. Ground Term Symbol For D5.
From www.chegg.com
Solved The following table lists the possible terms that Ground Term Symbol For D5 Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The ground state term symbol for d5 is 6s. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to. Ground Term Symbol For D5.
From www.vrogue.co
Solveddetermine The Ground State Term Symbol Of The A vrogue.co Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The answer to this question involves hund's rule, which make. Ground Term Symbol For D5.
From www.vrogue.co
Solveddetermine The Ground State Term Symbol Of The A vrogue.co Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Please explain how to get the answer with the necessary. Term correlation for d1, d9, d2, d8 configuration are shown completely while. Ground Term Symbol For D5.
From za.pinterest.com
grounding symbols Ground symbol, Electrical symbols, History lessons Ground Term Symbol For D5 Write the term symbol for the ground state. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3,. Ground Term Symbol For D5.
From www.slideserve.com
PPT Atomic Term Symbols PowerPoint Presentation, free download ID296390 Ground Term Symbol For D5 Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Write the term symbol for the ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. The answer. Ground Term Symbol For D5.
From www.slideserve.com
PPT Atomic Term Symbols PowerPoint Presentation, free download ID296390 Ground Term Symbol For D5 The ground state term symbol for d5 is 6s. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Please explain how to get the answer with the necessary. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Term correlation for. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). The ground state term symbol for d5 is 6s. This is because the five electrons in the d orbital can have six different spin states, and the total orbital. Ground Term Symbol For D5.
From www.teachmint.com
Rules To Find Ground Term Symbol Chemistry Notes Teachmint Ground Term Symbol For D5 Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially. Ground Term Symbol For D5.
From www.youtube.com
Term symbol for 3d4s5p configuration Term symbol for nonequivalent cofigGround state term Ground Term Symbol For D5 Please explain how to get the answer with the necessary. Write the term symbol for the ground state. The ground state term symbol for d5 is 6s. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Let's use. Ground Term Symbol For D5.
From www.chegg.com
Write the term symbol for the ground state of the Ground Term Symbol For D5 Write the term symbol for the ground state. Please explain how to get the answer with the necessary. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various. Ground Term Symbol For D5.
From www.scribd.com
Term Symbol PDF Atomic Orbital Electron Configuration Ground Term Symbol For D5 Please explain how to get the answer with the necessary. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Write the term symbol for the ground state. The ground state term symbol for d5 is 6s. This is. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Please explain how to get the answer with the necessary. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine. Ground Term Symbol For D5.
From www.coursehero.com
[Solved] Derive the ground state term symbol for the following elements and... Course Hero Ground Term Symbol For D5 Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Please explain how to get the answer with the necessary. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The ground state term symbol for d5 is 6s. The. Ground Term Symbol For D5.
From www.chegg.com
Solved The ground state term symbol for d5 high spin is Ground Term Symbol For D5 The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Write the term symbol for. Ground Term Symbol For D5.
From www.studypool.com
SOLUTION Term symbol for d1p1 ground state Studypool Ground Term Symbol For D5 The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. Let's use the. Ground Term Symbol For D5.
From brainly.in
For high spin d5 and d5s1 configurations, the ground state term symbols, respectively are (a Ground Term Symbol For D5 The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). Write the term symbol for the ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular. Ground Term Symbol For D5.
From www.numerade.com
What is the ground state term symbol for each of the following dn configurations 1. d2 ii. ds Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Please explain how to get the answer with the necessary. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The ground state term symbol for d5 is 6s. Term correlation for. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). This is because the five electrons in the d orbital can have six different spin. Ground Term Symbol For D5.
From www.slideserve.com
PPT PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular. Ground Term Symbol For D5.
From www.numerade.com
SOLVED The ground state term symbol for the free ion Fe3+ is Point) SD 65 6P 6D 4F 15 Ground Term Symbol For D5 Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). The ground state. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Please explain how to get the answer with the necessary. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The ground state term symbol for d5 is 6s. The answer to this question involves hund's rule, which make a lot more sense in. Ground Term Symbol For D5.
From www.vrogue.co
Solveddetermine The Ground State Term Symbol Of The A vrogue.co Ground Term Symbol For D5 Write the term symbol for the ground state. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Please explain how to get the answer with the necessary. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. This is. Ground Term Symbol For D5.
From www.youtube.com
Trick for Ground State Term Symbol for elements Physics and Chemistry YouTube Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Please explain how to get the answer with the necessary. Write the term symbol for the ground state. Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. The answer to this. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. Please explain how to get the answer with the necessary. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that are used to combine the various \(l\) and \(s\). The ground state term symbol. Ground Term Symbol For D5.
From www.youtube.com
Ground State Term Symbol for Transition Metal Complexes 3d Metal Ion Chemistry YouTube Ground Term Symbol For D5 This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Term correlation for d1, d9, d2, d8 configuration are shown completely while for d3, d4, d5, d6, d7 are shown partially by taking only lower energy. The answer to this question involves hund's rule, which make. Ground Term Symbol For D5.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Presentation ID2043186 Ground Term Symbol For D5 Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. The answer to this question involves hund's rule, which make a lot more sense in the context of generated term symbols that. Ground Term Symbol For D5.
From www.vrogue.co
Solveddetermine The Ground State Term Symbol Of The A vrogue.co Ground Term Symbol For D5 Let's use the example of the \(\ce{[cr(nh3)6]^3+}\) complex ion, and determine its ground state. This is because the five electrons in the d orbital can have six different spin states, and the total orbital angular momentum is 0. Write the term symbol for the ground state. The answer to this question involves hund's rule, which make a lot more sense. Ground Term Symbol For D5.