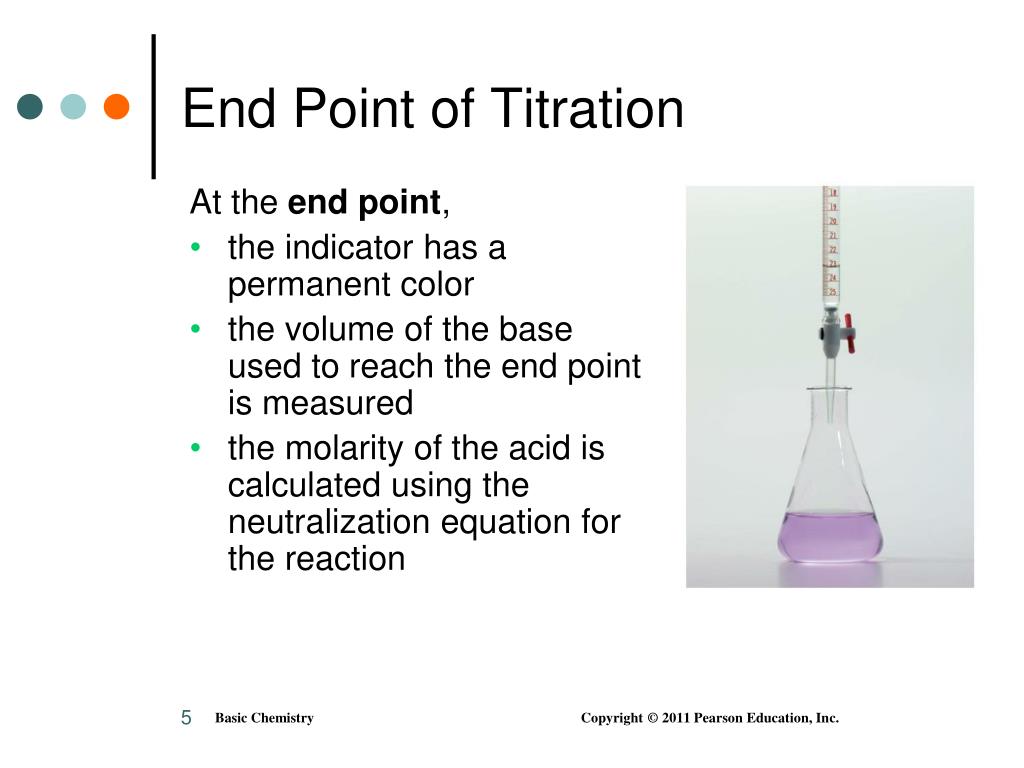
End Point Of Edta Titration Can Be Determined By . The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. Ph for edta titration of fe 3+ is slightly higher than 1.00. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The end point occurs when essentially all of the cation has reacted. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. A solution containing both ca 2+ and fe 3+. In this experiment you will standardize a solution of edta by titration. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity.
from www.slideserve.com
This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The end point occurs when essentially all of the cation has reacted. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. In this experiment you will standardize a solution of edta by titration. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. Ph for edta titration of fe 3+ is slightly higher than 1.00. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. A solution containing both ca 2+ and fe 3+.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
End Point Of Edta Titration Can Be Determined By The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. A solution containing both ca 2+ and fe 3+. In this experiment you will standardize a solution of edta by titration. Ph for edta titration of fe 3+ is slightly higher than 1.00. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The end point occurs when essentially all of the cation has reacted.
From slidetodoc.com
EDTA Titrations Introduction 1 Metal Chelate Complexes Any End Point Of Edta Titration Can Be Determined By The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. A solution containing both ca 2+ and fe 3+. Ph for edta titration of fe 3+ is slightly higher than 1.00. For example, we can identify the end point for a titration of cu 2 + with. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 End Point Of Edta Titration Can Be Determined By The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. In this experiment you will standardize a solution of edta by titration. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. The endpoint of the titration is determined by. End Point Of Edta Titration Can Be Determined By.
From www.animalia-life.club
Endpoint Titration End Point Of Edta Titration Can Be Determined By The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. The end point occurs when essentially all of the cation has reacted. For. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED "To standardize EDTA solution. three independent titrations End Point Of Edta Titration Can Be Determined By The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. In this experiment you will standardize a solution of edta by titration. Ph for edta titration of fe 3+ is slightly higher than 1.00. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount. End Point Of Edta Titration Can Be Determined By.
From www.youtube.com
Determination of Hardness by EDTA Method YouTube End Point Of Edta Titration Can Be Determined By The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. For example, we. End Point Of Edta Titration Can Be Determined By.
From www.youtube.com
Determination of Hardness by Complexometric/ EDTA Method_Water and its End Point Of Edta Titration Can Be Determined By The end point occurs when essentially all of the cation has reacted. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. A solution containing both ca 2+ and fe 3+. The overall procedure to be used involves the standardization of an edta. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID234018 End Point Of Edta Titration Can Be Determined By This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. A solution containing both ca 2+ and fe 3+. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The end point of the reaction is. End Point Of Edta Titration Can Be Determined By.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts End Point Of Edta Titration Can Be Determined By A solution containing both ca 2+ and fe 3+. The end point occurs when essentially all of the cation has reacted. Ph for edta titration of fe 3+ is slightly higher than 1.00. In this experiment you will standardize a solution of edta by titration. This article focuses on basic principles, titration curves, end point detection, titration types, and practical. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free End Point Of Edta Titration Can Be Determined By The end point occurs when essentially all of the cation has reacted. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. Ph for edta titration of fe 3+ is slightly higher than 1.00. A solution containing both ca 2+ and fe 3+. The overall procedure. End Point Of Edta Titration Can Be Determined By.
From www.youtube.com
V52 EDTA Titration Calcs YouTube End Point Of Edta Titration Can Be Determined By The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. Ph for edta titration of fe 3+ is slightly higher than 1.00. In this experiment you will standardize a solution of edta by titration. A solution containing both ca 2+ and fe 3+. For example, we. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations Chapter 13 PowerPoint Presentation, free download End Point Of Edta Titration Can Be Determined By For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. Ph for edta titration of fe 3+ is slightly higher than 1.00. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. The end point occurs. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 End Point Of Edta Titration Can Be Determined By For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The end point of the reaction is indicated by the formation of a permanent precipitate or. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED The endpoint of an EDTA titration is determined with End Point Of Edta Titration Can Be Determined By The end point occurs when essentially all of the cation has reacted. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. In this experiment you will standardize a solution of edta by titration. The overall procedure to be used involves the standardization of an edta. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 End Point Of Edta Titration Can Be Determined By Ph for edta titration of fe 3+ is slightly higher than 1.00. The end point occurs when essentially all of the cation has reacted. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. A solution containing both ca 2+ and fe 3+. In this experiment you will standardize a solution of edta. End Point Of Edta Titration Can Be Determined By.
From slideplayer.com
Chapter 11 EDTA Titrations ppt download End Point Of Edta Titration Can Be Determined By In this experiment you will standardize a solution of edta by titration. A solution containing both ca 2+ and fe 3+. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The end point occurs when essentially all of the cation has reacted.. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED 2 The amount of Ca in blood serum is determined by End Point Of Edta Titration Can Be Determined By In this experiment you will standardize a solution of edta by titration. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. For example, we can. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED The selectivity of EDTA titrations. The following can be End Point Of Edta Titration Can Be Determined By The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The end point of the reaction is indicated by. End Point Of Edta Titration Can Be Determined By.
From slidetodoc.com
EDTA Titrations Chelation in Biochemistry Chelating ligands can End Point Of Edta Titration Can Be Determined By The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. The end point occurs when essentially all of the. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED 3 Back Titration Ni?+ was determined in a complexometric End Point Of Edta Titration Can Be Determined By In this experiment you will standardize a solution of edta by titration. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. A solution containing both ca 2+ and fe 3+. Ph for edta titration of fe 3+ is slightly higher than 1.00.. End Point Of Edta Titration Can Be Determined By.
From www.numerade.com
SOLVED You are planning to perform an EDTA titration of solution End Point Of Edta Titration Can Be Determined By The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The endpoint of the titration is determined by the addition of eriochrome black t, which forms. End Point Of Edta Titration Can Be Determined By.
From slidetodoc.com
EDTA Titrations Introduction 1 Metal Chelate Complexes Any End Point Of Edta Titration Can Be Determined By This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. A solution containing both ca 2+ and fe 3+. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. Ph for edta titration of fe 3+ is slightly higher. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Of Edta Titration Can Be Determined By Ph for edta titration of fe 3+ is slightly higher than 1.00. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The end point occurs when essentially all of the cation has reacted. For example, we can identify the end point for a titration of. End Point Of Edta Titration Can Be Determined By.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube End Point Of Edta Titration Can Be Determined By This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. A solution containing both ca 2+ and fe 3+. For example, we can identify the end point for a titration. End Point Of Edta Titration Can Be Determined By.
From slideplayer.com
Complexometric titration Dr.Bhagure G.R. ppt download End Point Of Edta Titration Can Be Determined By The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. A solution containing both ca 2+ and fe 3+. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. The overall procedure to be used involves. End Point Of Edta Titration Can Be Determined By.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID234018 End Point Of Edta Titration Can Be Determined By The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. For example, we can identify the end point for. End Point Of Edta Titration Can Be Determined By.
From www.researchgate.net
How to determine the end point of EDTA titration? End Point Of Edta Titration Can Be Determined By In this experiment you will standardize a solution of edta by titration. Ph for edta titration of fe 3+ is slightly higher than 1.00. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. For example, we can identify the end point for a titration of cu. End Point Of Edta Titration Can Be Determined By.
From www.chegg.com
Solved 2 Ca EDTA titration Both precipitation and End Point Of Edta Titration Can Be Determined By In this experiment you will standardize a solution of edta by titration. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. A solution containing both. End Point Of Edta Titration Can Be Determined By.
From slidetodoc.com
EDTA Titrations Introduction 1 Metal Chelate Complexes Any End Point Of Edta Titration Can Be Determined By This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount. End Point Of Edta Titration Can Be Determined By.
From www.scribd.com
EDTA Titration PDF Titration Chemistry End Point Of Edta Titration Can Be Determined By A solution containing both ca 2+ and fe 3+. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a. End Point Of Edta Titration Can Be Determined By.
From mungfali.com
EDTA Titration Curve End Point Of Edta Titration Can Be Determined By Ph for edta titration of fe 3+ is slightly higher than 1.00. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. The endpoint of the titration is determined by. End Point Of Edta Titration Can Be Determined By.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications End Point Of Edta Titration Can Be Determined By The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. For example, we can identify the end point for a titration of cu. End Point Of Edta Titration Can Be Determined By.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre End Point Of Edta Titration Can Be Determined By For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The overall procedure to be used. End Point Of Edta Titration Can Be Determined By.
From in.pinterest.com
End Point Flashcard 10th Grade Science Self help book, Easy science End Point Of Edta Titration Can Be Determined By The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. Ph for edta titration of fe 3+ is slightly higher than 1.00. For example, we can identify the end point for a titration of cu 2 + with edta in the presence of nh 3 by monitoring the titrand’s. The end point occurs. End Point Of Edta Titration Can Be Determined By.
From slidetodoc.com
Chapter 12 EDTA Titrations Overview 12 1 MetalChelate End Point Of Edta Titration Can Be Determined By For example, we can identify the end point for a titration of cu 2 + with edta, in the presence of nh 3 by monitoring the titrand’s. The endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. The end point of the reaction is indicated by. End Point Of Edta Titration Can Be Determined By.
From slideplayer.com
Chapter 11 EDTA Titrations ppt download End Point Of Edta Titration Can Be Determined By A solution containing both ca 2+ and fe 3+. The end point occurs when essentially all of the cation has reacted. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity. The endpoint of the titration. End Point Of Edta Titration Can Be Determined By.