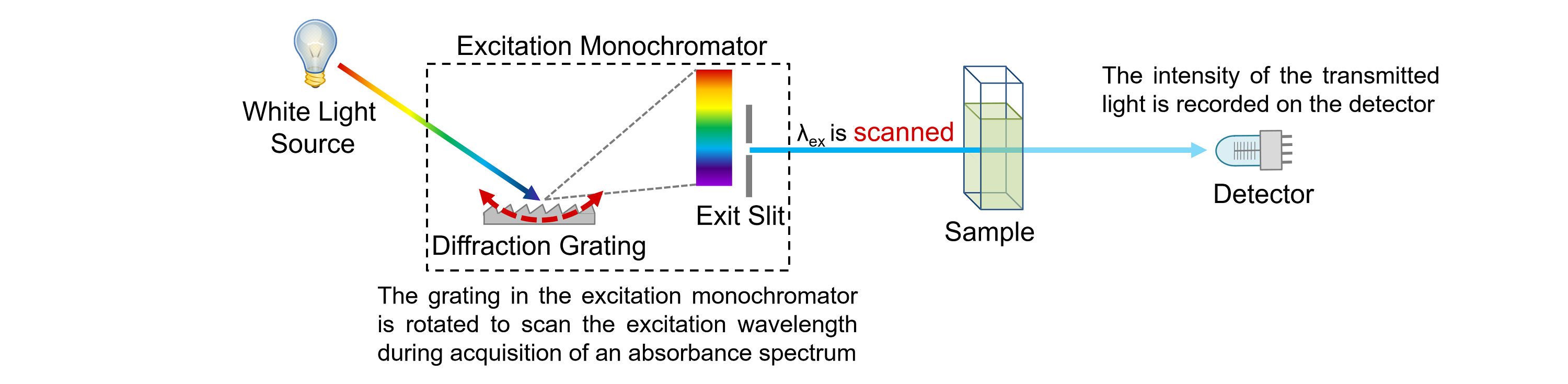
Spectroscopy Wavelength Absorbance . For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. The key components of a spectrophotometer are: A spectrophotometer in an instrument that measures the amount of light absorbed at a. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of.
from shaniyaukung.blogspot.com
The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The key components of a spectrophotometer are: For a single wavelength, a is absorbance (unitless, usually seen as arb. A spectrophotometer in an instrument that measures the amount of light absorbed at a. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample.
What Is Absorbance In Spectrophotometer
Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. For a single wavelength, a is absorbance (unitless, usually seen as arb. The key components of a spectrophotometer are: A spectrophotometer in an instrument that measures the amount of light absorbed at a. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of.
From endurancelasers.com
Absorption wavelength spectrum for different materials glass, metal Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample.. Spectroscopy Wavelength Absorbance.
From users.highland.edu
Atomic Spectra and Models of the Atom Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. A spectrophotometer in an instrument that measures the amount of light absorbed at a. An absorption spectrum will show a number of absorption bands corresponding to structural. Spectroscopy Wavelength Absorbance.
From chemistry.stackexchange.com
uv vis spectroscopy Absorption wavelength of nickel (II) Chemistry Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. A spectrophotometer in an instrument that measures the amount of light absorbed at a. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on. Spectroscopy Wavelength Absorbance.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. The simplest form of spectroscopy is absorption, which measures how much light of a given. Spectroscopy Wavelength Absorbance.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is. Spectroscopy Wavelength Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. An absorption spectrum will show a number of absorption bands corresponding to structural. Spectroscopy Wavelength Absorbance.
From www.thoughtco.com
Balmer Series Definition in Science Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the. Spectroscopy Wavelength Absorbance.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. For a single wavelength, a is absorbance (unitless, usually seen as arb. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration. Spectroscopy Wavelength Absorbance.
From shaniyaukung.blogspot.com
What Is Absorbance In Spectrophotometer Spectroscopy Wavelength Absorbance For a single wavelength, a is absorbance (unitless, usually seen as arb. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. The key components of a spectrophotometer are: An absorption spectrum will. Spectroscopy Wavelength Absorbance.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Wavelength Absorbance An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. A spectrophotometer in an instrument that measures the amount of light absorbed at a. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. The simplest form. Spectroscopy Wavelength Absorbance.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. For a single wavelength, a is absorbance (unitless, usually seen as arb. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is. Spectroscopy Wavelength Absorbance.
From www.newport.com.cn
IR Absorption Spectroscopy Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. A spectrophotometer in an instrument that measures the amount of light absorbed. Spectroscopy Wavelength Absorbance.
From www.jove.com
UltravioletVisible (UVVis) Spectroscopy Definition JoVE Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
Ultraviolet absorbance spectra. (A) Absorbance spectra of PDCe and the Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
Normalized absorbance and emission intensity spectra versus wavelength Spectroscopy Wavelength Absorbance An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. A spectrophotometer in an instrument that. Spectroscopy Wavelength Absorbance.
From fleminglaser.com.au
Absorption Spectrum (A2) Fleming Laser Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. The key components of a spectrophotometer are: An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The simplest form of spectroscopy is absorption, which measures. Spectroscopy Wavelength Absorbance.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Spectroscopy Wavelength Absorbance When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. A spectrophotometer. Spectroscopy Wavelength Absorbance.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Spectroscopy Wavelength Absorbance When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. For a single wavelength, a is absorbance (unitless, usually seen as arb. A spectrophotometer in an instrument that measures the amount of light absorbed at a. The simplest form of spectroscopy is absorption, which. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
UVvis absorption spectra of absorbance versus wavelength from 200e750 Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. A spectrophotometer in an instrument that measures. Spectroscopy Wavelength Absorbance.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectroscopy Wavelength Absorbance An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. Units or arbitrary units), ε. Spectroscopy Wavelength Absorbance.
From quizdbrelighting.z4.web.core.windows.net
How To Measure Absorbance Spectroscopy Wavelength Absorbance An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. A spectrophotometer in an instrument that measures the amount of light absorbed at a. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. The simplest. Spectroscopy Wavelength Absorbance.
From courses.lumenlearning.com
Spectrums of Light Biology for Majors I Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. For a single wavelength, a. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
UVVisible spectra for platinum nanoparticles, showing absorbance peak Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. For a single wavelength, a is absorbance (unitless, usually seen as arb. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The key components of a spectrophotometer are: Units or arbitrary units), ε is the molar absorptivity of. Spectroscopy Wavelength Absorbance.
From ar.inspiredpencil.com
Absorption Spectrum Of Chlorophyll A And B And Carotenoids Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. A spectrophotometer in an instrument that. Spectroscopy Wavelength Absorbance.
From www.pinterest.co.uk
The Red Shift of a Hydrogen Absorption Spectrum Wednesday, March 28 Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. A spectrophotometer in an instrument that measures the amount of light absorbed at a. For a single wavelength, a is absorbance (unitless, usually seen as arb. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. The simplest. Spectroscopy Wavelength Absorbance.
From www.slideserve.com
PPT Higher Biology PowerPoint Presentation, free download ID366128 Spectroscopy Wavelength Absorbance For a single wavelength, a is absorbance (unitless, usually seen as arb. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. A spectrophotometer in an instrument that measures the amount of light absorbed at a. The key components of a spectrophotometer are: The. Spectroscopy Wavelength Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Spectroscopy Wavelength Absorbance The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb.. Spectroscopy Wavelength Absorbance.
From rheolution.com
Understanding Absorbance at Specific Wavelengths Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. The key components of a spectrophotometer are: A spectrophotometer in an instrument that measures the amount of light absorbed at a. An absorption spectrum will show a number of absorption bands corresponding to. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
Wavelength absorbance spectrum of the two phases of a biphasic Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. A spectrophotometer in an instrument that measures the amount of light absorbed at a. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. The key components of a spectrophotometer are: When we. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
UV absorbance spectra of phenol as a function of the photodegradation Spectroscopy Wavelength Absorbance A spectrophotometer in an instrument that measures the amount of light absorbed at a. The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. For a single wavelength, a is absorbance (unitless, usually seen as arb. Units or arbitrary units), ε is the molar absorptivity of the compound. Spectroscopy Wavelength Absorbance.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. For a single wavelength, a is absorbance (unitless, usually seen as arb.. Spectroscopy Wavelength Absorbance.
From www.animalia-life.club
Wavelength Color Chart Spectroscopy Wavelength Absorbance When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. For a single wavelength, a is absorbance (unitless, usually seen as arb. An absorption spectrum will show a number. Spectroscopy Wavelength Absorbance.
From www.researchgate.net
UVVis spectrum (black arrows shows peak absorbance at 420 nm in the Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. The key components of a spectrophotometer are: When we use a spectroscopic method to measure the concentration of a sample, we select out a specific wavelength of radiation to shine on the sample. For a single wavelength, a is absorbance (unitless, usually seen as arb.. Spectroscopy Wavelength Absorbance.
From www.vernier.com
Decoding Your Absorbance Readings Vernier Spectroscopy Wavelength Absorbance Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. A spectrophotometer in an instrument that measures the amount of light absorbed at a. The key components of a spectrophotometer are: For a single wavelength, a is absorbance (unitless, usually seen as arb. The simplest form of spectroscopy is absorption, which measures how much light. Spectroscopy Wavelength Absorbance.
From www.color-meanings.com
The Visible Spectrum Overview With Colors Listed in Order of Spectroscopy Wavelength Absorbance The simplest form of spectroscopy is absorption, which measures how much light of a given frequency is absorbed by a collection of. Units or arbitrary units), ε is the molar absorptivity of the compound or molecule in. An absorption spectrum will show a number of absorption bands corresponding to structural groups within the molecule. For a single wavelength, a is. Spectroscopy Wavelength Absorbance.