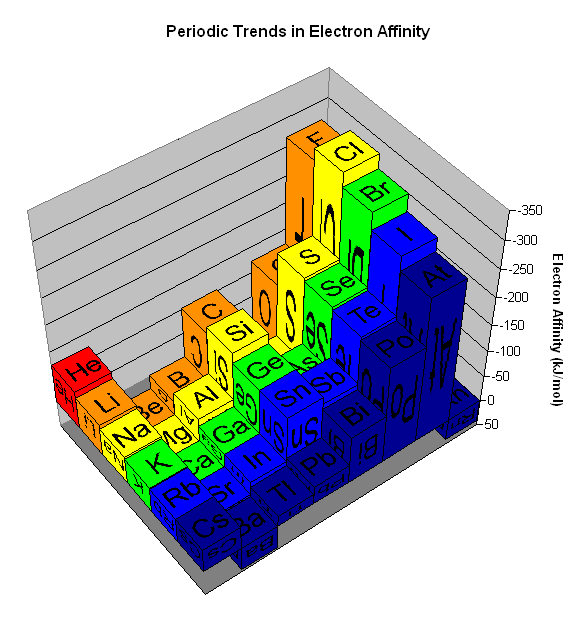
Lower Electron Affinity Than Chlorine . Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. This makes the fluoride anion so. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Consequently, the elements of the third row (n = 3) have the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Most metals have lower electron affinity values. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Which element has the lowest electron affinity? If this is the case,.
from chemistry.stackexchange.com
Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Consequently, the elements of the third row (n = 3) have the. This makes the fluoride anion so. If this is the case,. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Most metals have lower electron affinity values. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Which element has the lowest electron affinity?
chemistry If Oxygen has a lower electron affinity than
Lower Electron Affinity Than Chlorine I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Most metals have lower electron affinity values. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. If this is the case,. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. Which element has the lowest electron affinity? Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Consequently, the elements of the third row (n = 3) have the. This makes the fluoride anion so. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size.
From www.youtube.com
Electron affinity of chlorine is higher than that of flourine YouTube Lower Electron Affinity Than Chlorine This makes the fluoride anion so. Which element has the lowest electron affinity? Consequently, the elements of the third row (n = 3) have the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine.. Lower Electron Affinity Than Chlorine.
From loespjldd.blob.core.windows.net
Chlorine Electron Affinity Value at Timothy Peterson blog Lower Electron Affinity Than Chlorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Most metals have lower electron affinity values. Metals have a low electron affinity (a less likely chance to gain electrons) because they want. Lower Electron Affinity Than Chlorine.
From chemistry.stackexchange.com
chemistry If Oxygen has a lower electron affinity than Lower Electron Affinity Than Chlorine Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Consequently, the elements of the third row (n = 3) have the. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Which element. Lower Electron Affinity Than Chlorine.
From study.com
Electron Affinity Definition, Trends & Equation Video & Lesson Lower Electron Affinity Than Chlorine Consequently, the elements of the third row (n = 3) have the. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. This makes the fluoride anion so. If this is the case,. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Metals have a. Lower Electron Affinity Than Chlorine.
From www.youtube.com
Why Electron Affinity of Fluorine is lower than Chlorine by Tariq Lower Electron Affinity Than Chlorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. This makes the fluoride anion so. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. Metals have a low electron affinity (a less likely chance to gain electrons) because they want to. Lower Electron Affinity Than Chlorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Lower Electron Affinity Than Chlorine 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Fluorine, though higher than chlorine in the periodic table, has a very. Lower Electron Affinity Than Chlorine.
From www.youtube.com
, The lower electron affinity of fluorine than that of chlorine is due Lower Electron Affinity Than Chlorine 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. If this is the case,. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Metals have a low electron affinity (a less likely chance to gain electrons) because they. Lower Electron Affinity Than Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lower Electron Affinity Than Chlorine If this is the case,. Consequently, the elements of the third row (n = 3) have the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density.. Lower Electron Affinity Than Chlorine.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Lower Electron Affinity Than Chlorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. Consequently, the elements of the third row (n = 3) have the. Most metals have lower electron affinity values. I understand. Lower Electron Affinity Than Chlorine.
From periodictable.me
How To Find The Electron Configuration For Chlorine Lower Electron Affinity Than Chlorine This makes the fluoride anion so. Consequently, the elements of the third row (n = 3) have the. Which element has the lowest electron affinity? If this is the case,. Most metals have lower electron affinity values. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely. Lower Electron Affinity Than Chlorine.
From loespjldd.blob.core.windows.net
Chlorine Electron Affinity Value at Timothy Peterson blog Lower Electron Affinity Than Chlorine Consequently, the elements of the third row (n = 3) have the. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. 119 rows electron affinity is the amount of energy change (δe). Lower Electron Affinity Than Chlorine.
From ecurrencythailand.com
What Is Fluorine Electron Affinity? All Answers Lower Electron Affinity Than Chlorine Which element has the lowest electron affinity? Fluorine, therefore, has a lower affinity for an added electron than does chlorine. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Most metals have lower electron affinity values. Fluorine is much more reactive than chlorine (despite the lower electron affinity). Lower Electron Affinity Than Chlorine.
From loespjldd.blob.core.windows.net
Chlorine Electron Affinity Value at Timothy Peterson blog Lower Electron Affinity Than Chlorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Lower Electron Affinity Than Chlorine.
From www.numerade.com
SOLVED What is true of electron affinity? It is equal for sodium and Lower Electron Affinity Than Chlorine This makes the fluoride anion so. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Consequently, the elements of the third row (n = 3) have the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size.. Lower Electron Affinity Than Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lower Electron Affinity Than Chlorine I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. This makes the fluoride anion so. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. 119 rows. Lower Electron Affinity Than Chlorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Lower Electron Affinity Than Chlorine Consequently, the elements of the third row (n = 3) have the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Which element has the lowest electron affinity? I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its. Lower Electron Affinity Than Chlorine.
From lasemsources.weebly.com
Electron affinity chart lasemsources Lower Electron Affinity Than Chlorine Most metals have lower electron affinity values. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius. Lower Electron Affinity Than Chlorine.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Lower Electron Affinity Than Chlorine This makes the fluoride anion so. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Most metals have lower electron affinity values. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in. Lower Electron Affinity Than Chlorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Lower Electron Affinity Than Chlorine If this is the case,. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Which element has the lowest electron affinity? Fluorine is much more reactive than chlorine (despite the lower electron affinity). Lower Electron Affinity Than Chlorine.
From www.doubtnut.com
The lower electron affinity of fluorine than that of chlorine is due t Lower Electron Affinity Than Chlorine Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. If this is the case,. Consequently, the elements of the third row (n = 3) have the. This makes the fluoride anion so. Fluorine is much more reactive than chlorine (despite the lower electron affinity). Lower Electron Affinity Than Chlorine.
From www.youtube.com
The lower electron affinity of fluorine than that of chlorine is due to Lower Electron Affinity Than Chlorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Which element has the lowest electron affinity? Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. I have read that fluorine has. Lower Electron Affinity Than Chlorine.
From www.nagwa.com
Question Video Identifying the Explanation for the Anomalous Electron Lower Electron Affinity Than Chlorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. This makes the fluoride anion so. Most metals have lower electron. Lower Electron Affinity Than Chlorine.
From socratic.org
Which following pairs of atoms, have a lower electron affinity? a) Ca,K Lower Electron Affinity Than Chlorine This makes the fluoride anion so. Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. If this is the case,. Consequently, the elements of the third row (n = 3) have the. 119 rows electron affinity is the amount of energy change (δe) that. Lower Electron Affinity Than Chlorine.
From 88guru.com
Electron Affinity Definition and Trends in Periodic Table Lower Electron Affinity Than Chlorine 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Consequently, the elements of the third row (n = 3) have the. Which element has the lowest electron affinity? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the. Lower Electron Affinity Than Chlorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Lower Electron Affinity Than Chlorine Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Most metals have lower electron affinity values. This makes the fluoride anion so. I have read that fluorine has a. Lower Electron Affinity Than Chlorine.
From www.collegesearch.in
Electron Affinity Factors Affecting, Halogens, Periodic Trends, Metals Lower Electron Affinity Than Chlorine Consequently, the elements of the third row (n = 3) have the. Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Most metals have lower electron affinity values. Which element has the lowest electron affinity? This makes the fluoride anion so. I understand that. Lower Electron Affinity Than Chlorine.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Lower Electron Affinity Than Chlorine If this is the case,. Consequently, the elements of the third row (n = 3) have the. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Which element has the lowest electron affinity? 119 rows. Lower Electron Affinity Than Chlorine.
From www.numerade.com
SOLVED On the basis of electron configurations, explain why sulfur has Lower Electron Affinity Than Chlorine If this is the case,. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Consequently, the elements of the third row (n = 3) have the. This makes the. Lower Electron Affinity Than Chlorine.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Lower Electron Affinity Than Chlorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. This makes the fluoride anion so. Most metals have lower electron affinity values. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. If this is the case,. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the. Lower Electron Affinity Than Chlorine.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Lower Electron Affinity Than Chlorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. 119 rows electron affinity is the amount of energy change (δe). Lower Electron Affinity Than Chlorine.
From www.chegg.com
Solved Explain why S has a lower electron affinity than Cl Lower Electron Affinity Than Chlorine 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Which element has the lowest electron affinity? Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Consequently,. Lower Electron Affinity Than Chlorine.
From www.collegesearch.in
Electron Affinity Factors Affecting, Halogens, Periodic Trends, Metals Lower Electron Affinity Than Chlorine Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain. Consequently, the elements of the third row (n = 3) have the. Most metals have lower electron affinity values. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic. Lower Electron Affinity Than Chlorine.
From www.chegg.com
Solved Noble gases have low electron affinity Lower Electron Affinity Than Chlorine I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. Consequently, the elements of the third row (n = 3) have the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. I have read that fluorine has a lower electron affinity than chlorine. Lower Electron Affinity Than Chlorine.
From ar.inspiredpencil.com
Electron Affinity List Lower Electron Affinity Than Chlorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. If this is the case,. I have read that fluorine has a lower electron affinity than chlorine despite its lower atomic radius because its electron cloud is extremely dense. Which element has the lowest electron affinity? Fluorine, therefore,. Lower Electron Affinity Than Chlorine.
From www.chegg.com
Solved Calcium has a greater electron affinity than Chlorine Lower Electron Affinity Than Chlorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. I understand that fluorine has a lower electron affinity than chlorine due to fluorine's compact size and higher negative charge density. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. Lower Electron Affinity Than Chlorine.