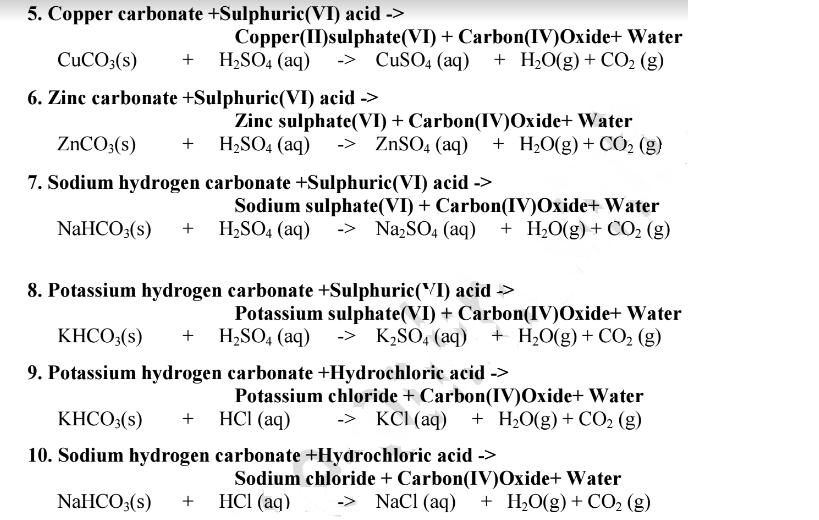
Magnesium Hydrogen Carbonate + Sulfuric Acid . To balance a chemical equation,. The symbol for magnesium is. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids will react with reactive metals, such as magnesium. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Acids react with metals, bases and carbonates to produce salts. Acid reactions with carbonates and hydrogencarbonates. Neutralisation is the reaction between an acid and a base. The easiest way to see this. Acids react with metal carbonates and hydrogencarbonates in the. To name them, follow these quick, simple rules: Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
from www.advance-africa.com
The easiest way to see this. To balance a chemical equation,. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Neutralisation is the reaction between an acid and a base. Acid reactions with carbonates and hydrogencarbonates. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Acids react with metals, bases and carbonates to produce salts. To name them, follow these quick, simple rules: Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3).
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests
Magnesium Hydrogen Carbonate + Sulfuric Acid Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Acids will react with reactive metals, such as magnesium. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids react with metal carbonates and hydrogencarbonates in the. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! To balance a chemical equation,. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Acid reactions with carbonates and hydrogencarbonates. The symbol for magnesium is. Neutralisation is the reaction between an acid and a base. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Acids react with metals, bases and carbonates to produce salts. The easiest way to see this. To name them, follow these quick, simple rules: Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3].
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.21 Practical Investigate Reactions Between Dilute Hydrochloric and Magnesium Hydrogen Carbonate + Sulfuric Acid Neutralisation is the reaction between an acid and a base. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). To balance a chemical equation,. Acid reactions with carbonates and hydrogencarbonates. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Use the calculator below to balance chemical equations and determine the type. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVED Magnesium reacts with sulfuric acid according to the following equation. How many moles Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). To balance a chemical equation,. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideserve.com
PPT Carbonic Acid PowerPoint Presentation ID3183947 Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. The easiest way to see this. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). To name them, follow these quick, simple rules: Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Use the calculator below to balance chemical equations and determine the type of. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.toppr.com
Convert the following word equations into molecular equation and balance them. 1) Ammonium Magnesium Hydrogen Carbonate + Sulfuric Acid Neutralisation is the reaction between an acid and a base. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids react with metal carbonates and hydrogencarbonates in the. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Acid reactions with carbonates. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideshare.net
Acids and alkalis ppt1292425009 Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. Neutralisation is the reaction between an acid and a base. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Such acids include sulfuric acid (h 2 so 4) or carbonic acid. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From w20.b2m.cz
Mg Oh 2 H2so4 EDUCA Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. The easiest way to see this. Acids react with metal carbonates and hydrogencarbonates in the. The symbol for magnesium is. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids react with metals, bases and carbonates to produce salts. Acids will react with reactive metals, such as. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVED What is the symbol equation for magnesium + sulphuric acid > magnesium sulphate + hydrogen Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. The easiest way to see this. Acid reactions with carbonates and hydrogencarbonates. Neutralisation is the reaction between an acid and a base. Use the calculator below to balance chemical. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From hydrogenpotanezu.blogspot.com
Hydrogen Magnesium Hydrogen Carbonate Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Acids will react with reactive metals, such as magnesium. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.toppr.com
Q5.Write the chemical formula a) Sulphuric acid b) Hydrochloric acid 2 HCL c) Nitric acid d Magnesium Hydrogen Carbonate + Sulfuric Acid Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids will react with reactive metals, such as magnesium. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Acids react with metal carbonates and hydrogencarbonates in the. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.toppr.com
Convert the following word equations into molecular equation and balance them. 1) Ammonium Magnesium Hydrogen Carbonate + Sulfuric Acid The easiest way to see this. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Acid reactions with carbonates and hydrogencarbonates. To balance a chemical equation,. To name them, follow these quick, simple rules: Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. Acids will react with. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.chegg.com
Solved 1. Sodium hydrogen carbonate reacts with sulfuric Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideshare.net
Acids And Bases Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Acids react with metal carbonates and hydrogencarbonates in the. Acids react with metals,. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Magnesium Hydrogen Carbonate + Sulfuric Acid Acids will react with reactive metals, such as magnesium. To name them, follow these quick, simple rules: Acid reactions with carbonates and hydrogencarbonates. The symbol for magnesium is. Acids react with metals, bases and carbonates to produce salts. To balance a chemical equation,. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.youtube.com
Magnesium In Sulfuric Acid H2 gas popping test by H2SO4 and Mg in a single displacement Magnesium Hydrogen Carbonate + Sulfuric Acid Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Acids react with metal carbonates and hydrogencarbonates in the. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. The symbol for magnesium is. The easiest way. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From teachernotes4u.com
Magnesium sulfate is a salt. It can be prepared by the reaction between magnesium carbonate and Magnesium Hydrogen Carbonate + Sulfuric Acid The symbol for magnesium is. Neutralisation is the reaction between an acid and a base. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. To balance a chemical equation,. Acid reactions with carbonates and hydrogencarbonates. To name them, follow these quick, simple rules: Acids react with metal carbonates. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVED Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that Magnesium Hydrogen Carbonate + Sulfuric Acid The easiest way to see this. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). The symbol for magnesium is. Acids will react with reactive metals, such as magnesium. Acids react with metal carbonates and hydrogencarbonates in the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideserve.com
PPT ACIDS & BASES PowerPoint Presentation, free download ID2732225 Magnesium Hydrogen Carbonate + Sulfuric Acid To name them, follow these quick, simple rules: Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed.. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for the following reactions Magnesium Hydrogen Carbonate + Sulfuric Acid Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Such acids include sulfuric acid (h 2 so. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From slideplayer.com
Learning Objectives Acids and Alkalis ppt download Magnesium Hydrogen Carbonate + Sulfuric Acid Acids react with metals, bases and carbonates to produce salts. Acids will react with reactive metals, such as magnesium. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for H2SO4 + MgCO3 = MgSO4 + H2O + CO2 YouTube Magnesium Hydrogen Carbonate + Sulfuric Acid Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Acid reactions with carbonates and hydrogencarbonates. Acids react with metal carbonates and hydrogencarbonates in the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The easiest way to see this. The symbol for. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideserve.com
PPT Chemical Formulae and Balancing Chemical Equations PowerPoint Presentation ID2067720 Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. To name them, follow these quick, simple rules: Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! The easiest way to see this. Acid reactions with carbonates. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium Hydrogen Carbonate + Sulfuric Acid The symbol for magnesium is. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Neutralisation is the reaction between an acid and a base. The easiest way to see this. Acids will react with reactive metals, such as magnesium. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o +. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
Texts 1. Sulfuric acid and sodium carbonate H2SO4 + Na2CO3 > Na2SO4 + H2O + CO2 2. Sulfuric Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. The symbol for magnesium is. Acids react with metal carbonates and hydrogencarbonates in the. To name them, follow these quick, simple rules: Acids react with metals, bases and carbonates to produce salts. Acid reactions with carbonates and hydrogencarbonates. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.youtube.com
Sulfuric acid + magnesium YouTube Magnesium Hydrogen Carbonate + Sulfuric Acid To name them, follow these quick, simple rules: Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Acid reactions with carbonates and hydrogencarbonates. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Such acids include sulfuric acid (h 2 so 4) or. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideserve.com
PPT What You Will Learn Today PowerPoint Presentation, free download ID7041556 Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids react with metals, bases and carbonates to produce salts. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To name them, follow these quick, simple rules:. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From stock.adobe.com
Stockvideon Chemical reaction of dissolution of pieces of magnesium in dilute sulfuric acid. As Magnesium Hydrogen Carbonate + Sulfuric Acid Neutralisation is the reaction between an acid and a base. Acids react with metal carbonates and hydrogencarbonates in the. The easiest way to see this. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVEDIf 1.00 g of magnesium hydroxide reacts with 0.605 g of sulfuric acid, what is the mass Magnesium Hydrogen Carbonate + Sulfuric Acid Acid reactions with carbonates and hydrogencarbonates. Acids react with metal carbonates and hydrogencarbonates in the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The easiest way to see this. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Acids will. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.youtube.com
How to Balance Mg + H2SO4 = MgSO4 + SO2 + H2O (Magnesium + Concentrated Sulfuric acid ) YouTube Magnesium Hydrogen Carbonate + Sulfuric Acid To name them, follow these quick, simple rules: Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Acid reactions with carbonates and hydrogencarbonates. Acids react with metal carbonates and hydrogencarbonates in the. Acids react with metals, bases and carbonates to produce salts. Magnesium combined with sulfuric acid. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From hydrogenpotanezu.blogspot.com
Hydrogen Magnesium Hydrogen Carbonate Magnesium Hydrogen Carbonate + Sulfuric Acid Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where two moles of magnesium carbonate [mgco 3]. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To name them, follow these quick, simple rules: Acid reactions with carbonates and hydrogencarbonates. Magnesium readily reacts with sulfuric acid and forms hydrogen gas. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.echemi.com
What substance is produced when sulphuric acid reacts with both magnesium oxide and magnesium Magnesium Hydrogen Carbonate + Sulfuric Acid Neutralisation is the reaction between an acid and a base. To balance a chemical equation,. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids will react with reactive metals, such as magnesium. Acid reactions with carbonates and hydrogencarbonates. Mgco3 + h2so4. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.slideshare.net
Chemical Reactions Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. The symbol for magnesium is. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! To balance a chemical equation,. Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Magnesium Hydrogen Carbonate + Sulfuric Acid Neutralisation is the reaction between an acid and a base. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To name them, follow these quick, simple rules: Acids react with metal carbonates and hydrogencarbonates in the. Acids will react with reactive metals, such. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVED Sulfuric acid magnesium phosphate Carbonic acid lithium iodide MOLECULAR WEIGHT AND Magnesium Hydrogen Carbonate + Sulfuric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The symbol for magnesium is. To balance a chemical equation,. Magnesium combined with sulfuric acid produces magnesium sulfate and hydrogen gas. Acids react with metal carbonates and hydrogencarbonates in the. The easiest way to see this. Acid reactions with carbonates and hydrogencarbonates. Balance the reaction. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Magnesium Hydrogen Carbonate + Sulfuric Acid Magnesium readily reacts with sulfuric acid and forms hydrogen gas bubbles and aqueous magnesium sulfate after the reactants are consumed. Such acids include sulfuric acid (h 2 so 4) or carbonic acid (h 2 co 3). Acids react with metals, bases and carbonates to produce salts. Mgco3 + h2so4 = mg(hco3)2 + mgso4 is a double displacement (metathesis) reaction where. Magnesium Hydrogen Carbonate + Sulfuric Acid.
From w20.b2m.cz
Mg Oh 2 H2so4 EDUCA Magnesium Hydrogen Carbonate + Sulfuric Acid Acids react with metal carbonates and hydrogencarbonates in the. The easiest way to see this. Balance the reaction of mgco3 + caco3 + h2so4 = mgso4 + h2o + co2 + caso4 using this chemical equation balancer! Acids will react with reactive metals, such as magnesium. To name them, follow these quick, simple rules: Such acids include sulfuric acid (h. Magnesium Hydrogen Carbonate + Sulfuric Acid.