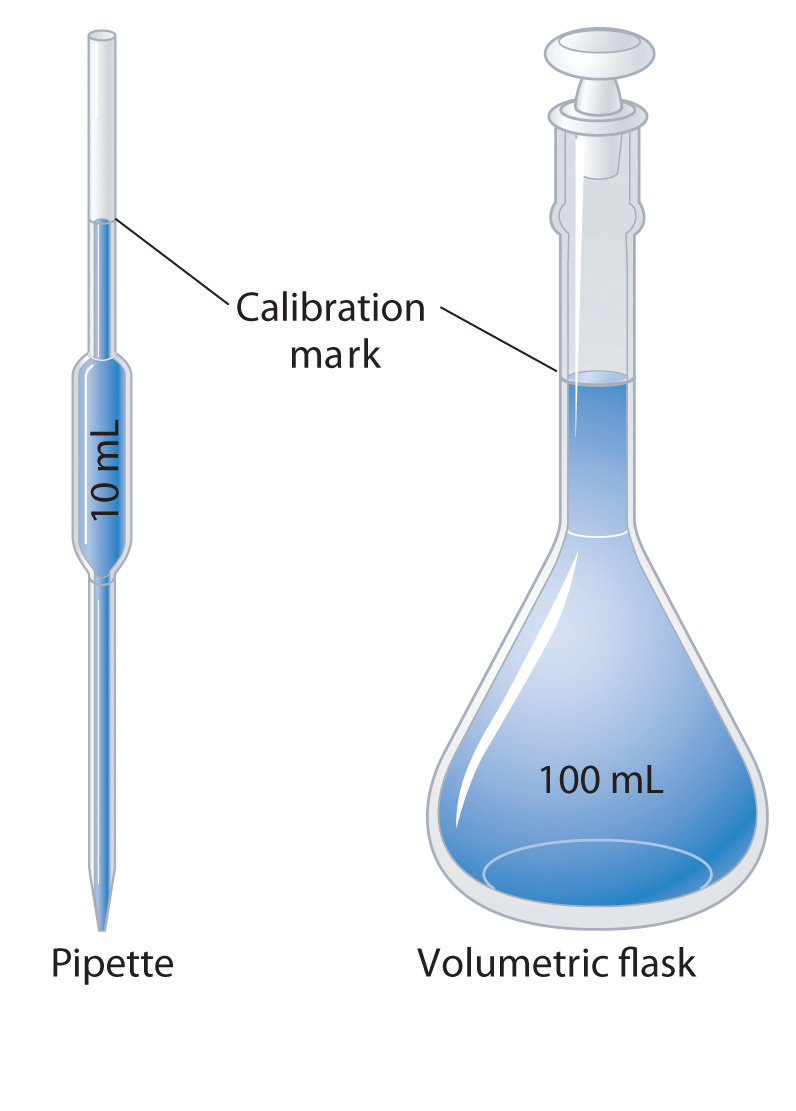
What Is The Uncertainty Of A 100 Ml Volumetric Flask . Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware should not be emptied by holding onto the neck alone. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250.
from saylordotorg.github.io
The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric glassware should not be emptied by holding onto the neck alone.
Essential Skills 1
What Is The Uncertainty Of A 100 Ml Volumetric Flask Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Volumetric glassware should not be emptied by holding onto the neck alone. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The bottom of the flask should always be supported. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric glassware, such as pipettes, burettes, and flasks, is required when.
From www.chegg.com
Solved 3. Using a 100mL volumetric flask, prepare an What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From ecatalog.corning.com
5642100 PYREX® 100 mL Class A Volumetric Flask with Polyethylene Standard Taper Stopper Corning What Is The Uncertainty Of A 100 Ml Volumetric Flask The bottom of the flask should always be supported. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.slideserve.com
PPT Laboratory Glassware PowerPoint Presentation, free download ID44627 What Is The Uncertainty Of A 100 Ml Volumetric Flask 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Volumetric glassware should not be emptied by holding onto the neck alone. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From whatishplc.com
Volumetric flask, everything you need to know about Expert Guide 👨🔬 What Is The Uncertainty Of A 100 Ml Volumetric Flask The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.gogenlab.com
Corning 5600100 PYREX® 100mL Class A Lifetime Red Volumetric Flask with Polyethylene SnapCap What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. 125 ml erlenmeyer flask 25 ml. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.smartscience.co.th
Plastic Volumetric Flask smartscience What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric glassware should not be emptied by holding onto the neck alone. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Uncertainty. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.indiamart.com
LABCO Borosilicate Glass 100ml Volumetric Flask with NABL Certificate, For Chemical Laboratory What Is The Uncertainty Of A 100 Ml Volumetric Flask The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From socratic.org
What is the tool that scientists commonly use to measure liquid volume? Socratic What Is The Uncertainty Of A 100 Ml Volumetric Flask Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. The bottom of the flask should always be supported. Uncertainty of measurement 10 ml graduated. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From glossary.periodni.com
Volumetric flask Chemistry Dictionary & Glossary What Is The Uncertainty Of A 100 Ml Volumetric Flask Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From pobel.com
VOLUMETRIC FLASK 100 ML FOR WINE DISTILLATION What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The bottom of the flask should always be supported. Using utmost care, the chemist can. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From medilabexports.com
Precision in Volumetric Flask Chemistry MEDILAB What Is The Uncertainty Of A 100 Ml Volumetric Flask The bottom of the flask should always be supported. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.eiscolabs.com
Volumetric Flask, 100ml Class A Tolerance ±0.10ml 14/23 Polypropyl — Eisco Labs What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.animalia-life.club
Volumetric Flask Laboratory Apparatus What Is The Uncertainty Of A 100 Ml Volumetric Flask Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. The bottom of the flask should always be supported. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Volumetric glassware should not be emptied. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.slideshare.net
Calibration of glassware What Is The Uncertainty Of A 100 Ml Volumetric Flask Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.norchemist.com
Volumetric Flask, Borosilicate Glass, 100 ml, Pack of 2 Norchemist What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). The bottom of the flask should always be supported. Volumetric glassware should not be emptied by holding onto the neck alone. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. 125 ml erlenmeyer. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.homesciencetools.com
100 ml Volumetric Flask Home Science Tools What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. The bottom of the flask should always be supported. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From gistgear.com
Best Lab Volumetric Flasks Buying Guide GistGear What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric glassware should not be emptied by holding onto the neck alone. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. Volumetric glassware, such as pipettes, burettes, and flasks, is. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From dxoywjwaa.blob.core.windows.net
Uncertainty Of 1 L Volumetric Flask at Eula Lewis blog What Is The Uncertainty Of A 100 Ml Volumetric Flask The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.chromatographyonline.com
Is the Solution Dilution? Hidden Uncertainty in Gas Chromatography Methods What Is The Uncertainty Of A 100 Ml Volumetric Flask 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware should not be emptied by holding onto the neck alone. Volumetric glassware, such as pipettes, burettes, and flasks, is. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From exogatbyy.blob.core.windows.net
Volumetric Flask Used In Titration at Jennifer Wetzler blog What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250.. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From saylordotorg.github.io
Essential Skills 1 What Is The Uncertainty Of A 100 Ml Volumetric Flask Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.youtube.com
Introduction to Volumetric Flask Types & Tips YouTube What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Volumetric glassware should not be emptied by holding onto the neck alone. Since the smallest division (graduation) is a tenth of. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From courses.lumenlearning.com
Measurement Uncertainty, Accuracy, and Precision CHEM 1305 Introductory Chemistry What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.numerade.com
SOLVED Table I airdisplacement pipette used, Uncertainty of airdisplacement pipette = 0.008 What Is The Uncertainty Of A 100 Ml Volumetric Flask The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.slideserve.com
PPT Session 2 Fundamentals and Classical methods of quantitative elemental analysis What Is The Uncertainty Of A 100 Ml Volumetric Flask Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Volumetric glassware should not be emptied by holding onto the neck alone. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. The overall uncertainty in the final concentration—and, therefore, the best option for. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From ar.inspiredpencil.com
Volumetric Flask What Is The Uncertainty Of A 100 Ml Volumetric Flask Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From adeniumbiotech.com
I Tested the Accuracy of a 100 ml Volumetric Flask and Here's What I Discovered! What Is The Uncertainty Of A 100 Ml Volumetric Flask 125 ml erlenmeyer flask 25 ml 1 25 ml ± 2.5 ml 250. The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Using utmost. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.numerade.com
SOLVED Calculate the combined standard uncertainty for the volume of isooctane in a 100 mL What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Uncertainty of measurement 10 ml graduated cylinder 1 ml 5 0.2 ml ± 0.02 ml. Volumetric glassware should not be emptied by holding onto the neck alone. Using utmost care, the chemist. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.numerade.com
Data Table 2 Determination of Uncertainty in Common Glassware Glassware Mass of empty glassware What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. The bottom of the flask should always be supported. Since the smallest division (graduation) is a tenth of a milliliter, we. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.thoughtco.com
What a Volumetric Flask Is and How to Use One What Is The Uncertainty Of A 100 Ml Volumetric Flask The overall uncertainty in the final concentration—and, therefore, the best option for the dilution—depends on the uncertainty of the. The bottom of the flask should always be supported. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Volumetric glassware, such as pipettes, burettes,. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.chegg.com
The uncertainty in the volumetric flasks is What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Volumetric glassware should not be emptied by holding onto the neck. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From labequipsupply.co.za
Volumetric Flask Lab Glassware Reflecta Laboratories What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Since the smallest division (graduation) is. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From studylib.net
volumetric flasks What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware, such as pipettes, burettes, and flasks, is required when. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.nagwa.com
Question Video Determining the Uncertainty in the Volume of a Mixture Nagwa What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Volumetric glassware should not be emptied by holding onto the neck alone. Using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume. What Is The Uncertainty Of A 100 Ml Volumetric Flask.
From www.progressive-scientific.com
Volumetric Flask IWAKI, Class A, w/Glass Stopper Progressive Scientific Sdn. Bhd. What Is The Uncertainty Of A 100 Ml Volumetric Flask Volumetric flasks transfer pipets flask capacity (ml) tolerance (ml) 1 0.02 2 0.02 5 0.02 10 0.02 25 0.03 50 0.05 100 0.08 200 0.10 250 0.12 500. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01). Volumetric glassware, such as pipettes, burettes, and flasks, is required when.. What Is The Uncertainty Of A 100 Ml Volumetric Flask.