Calorimetry Cheeto Combustion Lab . This procedure is known as. Energy content of food raw data from calorimetry analysis of cheeto samples table 2. Purpose the purpose of this experiment is to determine. By measuring the change in temperature. Lets investigate the caloric content of different snack foods such as marshmallows and. In this experiment we will burn a cheeto® and use the fire to heat water. when food is combusted, the reaction that occurs can be represented by the reaction equation: a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. how is the caloric content of food determined? A careful measurement of thermal energy released is required. The temperature change of the water,. determination of the caloric energy of a cheeto®. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat.
from classnotes.org.in
Purpose the purpose of this experiment is to determine. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. This procedure is known as. A careful measurement of thermal energy released is required. In this experiment we will burn a cheeto® and use the fire to heat water. Lets investigate the caloric content of different snack foods such as marshmallows and. determination of the caloric energy of a cheeto®. how is the caloric content of food determined? Energy content of food raw data from calorimetry analysis of cheeto samples table 2.
Measurement Of Change In Internal Energy and Enthalpy Chemistry
Calorimetry Cheeto Combustion Lab The temperature change of the water,. In this experiment we will burn a cheeto® and use the fire to heat water. when food is combusted, the reaction that occurs can be represented by the reaction equation: Energy content of food raw data from calorimetry analysis of cheeto samples table 2. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. This procedure is known as. A careful measurement of thermal energy released is required. Purpose the purpose of this experiment is to determine. Lets investigate the caloric content of different snack foods such as marshmallows and. determination of the caloric energy of a cheeto®. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. By measuring the change in temperature. how is the caloric content of food determined? The temperature change of the water,.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb Calorimetry Cheeto Combustion Lab how is the caloric content of food determined? Energy content of food raw data from calorimetry analysis of cheeto samples table 2. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. Purpose the purpose of this experiment is to determine. By measuring the change in temperature. Hydrocarbon (s) +. Calorimetry Cheeto Combustion Lab.
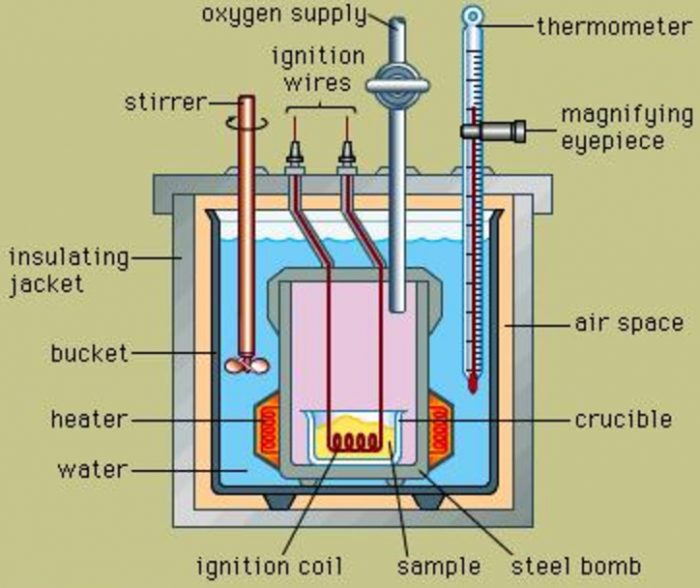
From www.laboratoryinstrumentindia.com
Mahler Calorimeter For Determining Heat Of Combustion Manufacturers Calorimetry Cheeto Combustion Lab This procedure is known as. In this experiment we will burn a cheeto® and use the fire to heat water. determination of the caloric energy of a cheeto®. Lets investigate the caloric content of different snack foods such as marshmallows and. Purpose the purpose of this experiment is to determine. a calorimeter is usually an isolated environment in. Calorimetry Cheeto Combustion Lab.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Calorimetry Cheeto Combustion Lab Lets investigate the caloric content of different snack foods such as marshmallows and. The temperature change of the water,. A careful measurement of thermal energy released is required. By measuring the change in temperature. how is the caloric content of food determined? determination of the caloric energy of a cheeto®. Hydrocarbon (s) + o2 (g) → co2 (g). Calorimetry Cheeto Combustion Lab.
From exyhgjebd.blob.core.windows.net
How Does A Bomb Calorimeter Measure Calories at Emma Sanchez blog Calorimetry Cheeto Combustion Lab The temperature change of the water,. A careful measurement of thermal energy released is required. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. By measuring the change in temperature. when food is combusted, the reaction that occurs can be represented by the reaction equation: Energy content of food raw data from calorimetry analysis of. Calorimetry Cheeto Combustion Lab.
From www.pinterest.co.uk
Pin on Science Friday Calorimetry Cheeto Combustion Lab By measuring the change in temperature. Purpose the purpose of this experiment is to determine. Lets investigate the caloric content of different snack foods such as marshmallows and. determination of the caloric energy of a cheeto®. Energy content of food raw data from calorimetry analysis of cheeto samples table 2. Hydrocarbon (s) + o2 (g) → co2 (g) +. Calorimetry Cheeto Combustion Lab.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Cheeto Combustion Lab Energy content of food raw data from calorimetry analysis of cheeto samples table 2. By measuring the change in temperature. how is the caloric content of food determined? The temperature change of the water,. when food is combusted, the reaction that occurs can be represented by the reaction equation: This procedure is known as. Purpose the purpose of. Calorimetry Cheeto Combustion Lab.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry Calorimetry Cheeto Combustion Lab when food is combusted, the reaction that occurs can be represented by the reaction equation: This procedure is known as. The temperature change of the water,. Lets investigate the caloric content of different snack foods such as marshmallows and. how is the caloric content of food determined? Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g). Calorimetry Cheeto Combustion Lab.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Cheeto Combustion Lab determination of the caloric energy of a cheeto®. how is the caloric content of food determined? This procedure is known as. A careful measurement of thermal energy released is required. Purpose the purpose of this experiment is to determine. when food is combusted, the reaction that occurs can be represented by the reaction equation: The temperature change. Calorimetry Cheeto Combustion Lab.
From www.studocu.com
Exp1 Bomb+Calorimetry A B Experiment 1 Determination of enthalpy of Calorimetry Cheeto Combustion Lab Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. A careful measurement of thermal energy released is required. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. This procedure is known as. Energy. Calorimetry Cheeto Combustion Lab.
From www.youtube.com
Enthalpy of Combustion Lab Explained YouTube Calorimetry Cheeto Combustion Lab A careful measurement of thermal energy released is required. By measuring the change in temperature. In this experiment we will burn a cheeto® and use the fire to heat water. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. how is the. Calorimetry Cheeto Combustion Lab.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Calorimetry Cheeto Combustion Lab determination of the caloric energy of a cheeto®. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. This procedure is known as. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. In this experiment we will burn a cheeto® and use the fire to heat. Calorimetry Cheeto Combustion Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Cheeto Combustion Lab Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Energy content of food raw data from calorimetry analysis of cheeto samples table 2. determination of the caloric energy of a cheeto®. how is the caloric content of food determined? A careful measurement of thermal energy released is required. Purpose the purpose of this experiment. Calorimetry Cheeto Combustion Lab.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter To Predict A Curve Calorimetry Cheeto Combustion Lab In this experiment we will burn a cheeto® and use the fire to heat water. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Energy content of food raw data from calorimetry analysis of cheeto samples table 2. By measuring the change in temperature. Purpose the purpose of this experiment is to determine. a calorimeter. Calorimetry Cheeto Combustion Lab.
From www.materials.imdea.org
New Fire Testing Instrument Microscale Combustion Calorimeter Calorimetry Cheeto Combustion Lab By measuring the change in temperature. how is the caloric content of food determined? This procedure is known as. In this experiment we will burn a cheeto® and use the fire to heat water. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. a calorimeter is usually an isolated environment in which the heat. Calorimetry Cheeto Combustion Lab.
From courses.lumenlearning.com
Calorimetry CHEM 1305 Introductory Chemistry Calorimetry Cheeto Combustion Lab how is the caloric content of food determined? The temperature change of the water,. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. when food is combusted, the reaction that occurs can be represented by the reaction equation: determination of. Calorimetry Cheeto Combustion Lab.
From www.studocu.com
Exp1 Combustion bomb calorimeter Experiment 1 Avzianova/Bozzelli Bomb Calorimetry Cheeto Combustion Lab Lets investigate the caloric content of different snack foods such as marshmallows and. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. Purpose the purpose of this experiment is to determine. determination of the caloric energy of a cheeto®. By measuring the change in temperature. The temperature change of. Calorimetry Cheeto Combustion Lab.
From studylib.net
Heat of Combustion Lab Calorimetry Cheeto Combustion Lab Purpose the purpose of this experiment is to determine. determination of the caloric energy of a cheeto®. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. A careful measurement of thermal energy released is required. Lets investigate the caloric content of different snack foods such as marshmallows and. . Calorimetry Cheeto Combustion Lab.
From hxekkbifm.blob.core.windows.net
Bomb Calorimeter Working Animation at Debra Seery blog Calorimetry Cheeto Combustion Lab In this experiment we will burn a cheeto® and use the fire to heat water. when food is combusted, the reaction that occurs can be represented by the reaction equation: determination of the caloric energy of a cheeto®. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Energy content of food raw data from. Calorimetry Cheeto Combustion Lab.
From 2012books.lardbucket.org
Calorimetry Calorimetry Cheeto Combustion Lab a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. This procedure is known as. Energy content of food raw data from calorimetry analysis of cheeto samples table 2. A careful measurement of thermal energy released is required. The temperature change of the water,. In this experiment we will burn a. Calorimetry Cheeto Combustion Lab.
From studylib.net
CalorimetryCheetoEnergyLab Calorimetry Cheeto Combustion Lab determination of the caloric energy of a cheeto®. Purpose the purpose of this experiment is to determine. when food is combusted, the reaction that occurs can be represented by the reaction equation: This procedure is known as. A careful measurement of thermal energy released is required. Energy content of food raw data from calorimetry analysis of cheeto samples. Calorimetry Cheeto Combustion Lab.
From socratic.org
Question 5d3f9 + Example Calorimetry Cheeto Combustion Lab when food is combusted, the reaction that occurs can be represented by the reaction equation: Lets investigate the caloric content of different snack foods such as marshmallows and. In this experiment we will burn a cheeto® and use the fire to heat water. A careful measurement of thermal energy released is required. a calorimeter is usually an isolated. Calorimetry Cheeto Combustion Lab.
From fyottwotl.blob.core.windows.net
Calorimeter Diy at Mabel Rearick blog Calorimetry Cheeto Combustion Lab A careful measurement of thermal energy released is required. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. how is the caloric content of food determined? In this experiment we will burn a cheeto® and use the fire to heat water. The temperature change of the water,. Lets investigate. Calorimetry Cheeto Combustion Lab.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Cheeto Combustion Lab A careful measurement of thermal energy released is required. By measuring the change in temperature. when food is combusted, the reaction that occurs can be represented by the reaction equation: Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. a calorimeter. Calorimetry Cheeto Combustion Lab.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.3 Calorimetry翰林国际教育 Calorimetry Cheeto Combustion Lab when food is combusted, the reaction that occurs can be represented by the reaction equation: Lets investigate the caloric content of different snack foods such as marshmallows and. Purpose the purpose of this experiment is to determine. In this experiment we will burn a cheeto® and use the fire to heat water. Energy content of food raw data from. Calorimetry Cheeto Combustion Lab.
From ar.inspiredpencil.com
Heat Of Combustion Lab Calorimetry Cheeto Combustion Lab determination of the caloric energy of a cheeto®. when food is combusted, the reaction that occurs can be represented by the reaction equation: Energy content of food raw data from calorimetry analysis of cheeto samples table 2. By measuring the change in temperature. Purpose the purpose of this experiment is to determine. Hydrocarbon (s) + o2 (g) →. Calorimetry Cheeto Combustion Lab.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Calorimetry Cheeto Combustion Lab By measuring the change in temperature. A careful measurement of thermal energy released is required. This procedure is known as. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. how is the caloric content of food determined? determination of the caloric energy of a cheeto®. Lets investigate the caloric content of different snack foods. Calorimetry Cheeto Combustion Lab.
From www.slideshare.net
2 pa32 bomb calorimeter procedure Calorimetry Cheeto Combustion Lab Lets investigate the caloric content of different snack foods such as marshmallows and. how is the caloric content of food determined? Energy content of food raw data from calorimetry analysis of cheeto samples table 2. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. By measuring the change in temperature. The temperature change of the. Calorimetry Cheeto Combustion Lab.
From cesngzbc.blob.core.windows.net
Calorimetry Lab Burning A Cheeto at Leroy Doyle blog Calorimetry Cheeto Combustion Lab This procedure is known as. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. Purpose the purpose of this experiment is to determine. In this experiment we will burn a cheeto® and use the fire to heat water. how is the caloric. Calorimetry Cheeto Combustion Lab.
From giobxedsi.blob.core.windows.net
Calorimeter Experiment Calculation at Mabel Ballenger blog Calorimetry Cheeto Combustion Lab Energy content of food raw data from calorimetry analysis of cheeto samples table 2. Lets investigate the caloric content of different snack foods such as marshmallows and. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. determination of the caloric energy of a cheeto®. This procedure is known as. In this experiment we will burn. Calorimetry Cheeto Combustion Lab.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimetry Cheeto Combustion Lab A careful measurement of thermal energy released is required. This procedure is known as. when food is combusted, the reaction that occurs can be represented by the reaction equation: a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. how is the caloric content of food determined? determination. Calorimetry Cheeto Combustion Lab.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Calorimetry Cheeto Combustion Lab In this experiment we will burn a cheeto® and use the fire to heat water. how is the caloric content of food determined? This procedure is known as. when food is combusted, the reaction that occurs can be represented by the reaction equation: Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. By measuring. Calorimetry Cheeto Combustion Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Cheeto Combustion Lab By measuring the change in temperature. Purpose the purpose of this experiment is to determine. how is the caloric content of food determined? A careful measurement of thermal energy released is required. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. Hydrocarbon (s) + o2 (g) → co2 (g). Calorimetry Cheeto Combustion Lab.
From studylib.net
Cheeto Calorimetry Lab Calorimetry Cheeto Combustion Lab Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Lets investigate the caloric content of different snack foods such as marshmallows and. how is the caloric content of food determined? determination of the caloric energy of a cheeto®. A careful measurement of thermal energy released is required. The temperature change of the water,. . Calorimetry Cheeto Combustion Lab.
From exyqlxbwd.blob.core.windows.net
Calorimetry Fundamentals Instrumentation And Applications at Jeffrey Calorimetry Cheeto Combustion Lab Lets investigate the caloric content of different snack foods such as marshmallows and. A careful measurement of thermal energy released is required. how is the caloric content of food determined? determination of the caloric energy of a cheeto®. By measuring the change in temperature. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Purpose. Calorimetry Cheeto Combustion Lab.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Cheeto Combustion Lab when food is combusted, the reaction that occurs can be represented by the reaction equation: In this experiment we will burn a cheeto® and use the fire to heat water. Purpose the purpose of this experiment is to determine. Hydrocarbon (s) + o2 (g) → co2 (g) + h2o (g) + heat. Energy content of food raw data from. Calorimetry Cheeto Combustion Lab.