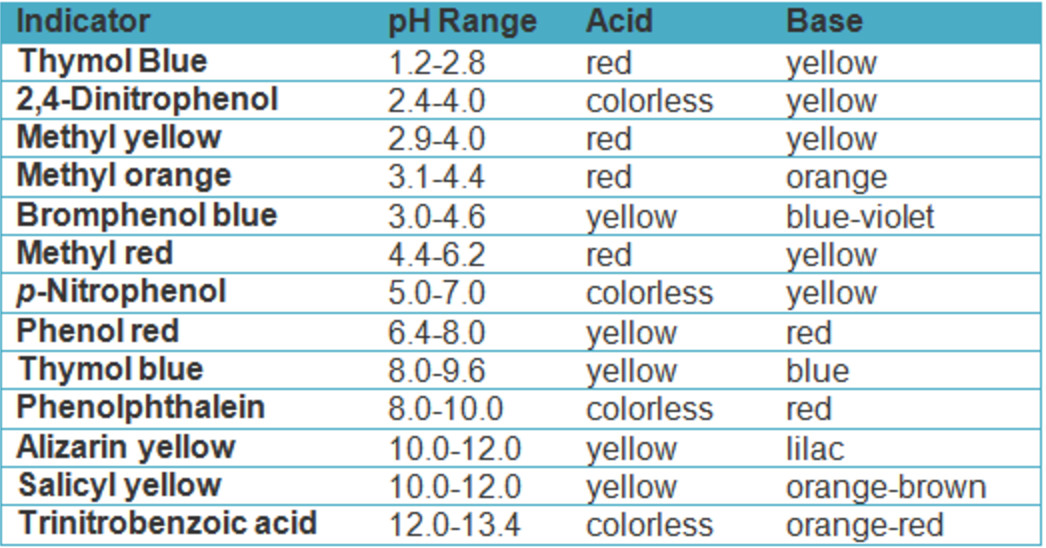
Best Indicator For Strong Base And Weak Acid . The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Weak acid v strong base. Here is a chart of common ph indicators, their ph range, their solutions,. Phenolphthalein is usually preferred because of its more easily seen colour change. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The correct answer is c. This time, the methyl orange is hopeless! However, the phenolphthalein changes colour exactly where you want it to. In the titration of a weak acid with a strong base, which indicator would be the best choice?
from dxokymive.blob.core.windows.net
The correct answer is c. This time, the methyl orange is hopeless! Weak acid v strong base. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Phenolphthalein is usually preferred because of its more easily seen colour change. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, which indicator would be the best choice? However, the phenolphthalein changes colour exactly where you want it to.
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog
Best Indicator For Strong Base And Weak Acid Here is a chart of common ph indicators, their ph range, their solutions,. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The correct answer is c. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Here is a chart of common ph indicators, their ph range, their solutions,. However, the phenolphthalein changes colour exactly where you want it to. Phenolphthalein is usually preferred because of its more easily seen colour change. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Weak acid v strong base. This time, the methyl orange is hopeless! Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, which indicator would be the best choice?
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry Best Indicator For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Here is a chart of common ph indicators, their ph range, their solutions,. This time, the methyl orange is hopeless! When titrating strong acids or bases, aim for a ph indicator that displays a color change. Best Indicator For Strong Base And Weak Acid.
From corbinkruwallen.blogspot.com
Strong Acid and Weak Acid CorbinkruwAllen Best Indicator For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Here is a chart of common ph indicators, their ph range, their solutions,. However, the phenolphthalein changes colour exactly where you want it to. Phenolphthalein is usually preferred because of its more easily seen colour change.. Best Indicator For Strong Base And Weak Acid.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Best Indicator For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Weak acid v strong base. However, the phenolphthalein changes colour exactly where you want it to. This time, the methyl orange. Best Indicator For Strong Base And Weak Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Best Indicator For Strong Base And Weak Acid This time, the methyl orange is hopeless! The correct answer is c. Phenolphthalein is usually preferred because of its more easily seen colour change. Any of the three indicators will exhibit. Weak acid v strong base. In the titration of a weak acid with a strong base, which indicator would be the best choice? When titrating strong acids or bases,. Best Indicator For Strong Base And Weak Acid.
From giorxivqj.blob.core.windows.net
Best Indicator For Hcl And Naoh Titration at Justin Leon blog Best Indicator For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. However, the phenolphthalein changes colour exactly where you want it to. In the titration of a weak acid with a strong base, which indicator would be the best choice? When titrating strong acids or bases, aim. Best Indicator For Strong Base And Weak Acid.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Best Indicator For Strong Base And Weak Acid Weak acid v strong base. However, the phenolphthalein changes colour exactly where you want it to. Here is a chart of common ph indicators, their ph range, their solutions,. In the titration of a weak acid with a strong base, which indicator would be the best choice? Any of the three indicators will exhibit. The titration of a weak acid. Best Indicator For Strong Base And Weak Acid.
From ar.inspiredpencil.com
Ph Examples Of Acids And Bases Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. The correct answer is c. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph indicator. Best Indicator For Strong Base And Weak Acid.
From www.vrogue.co
Differences Between Acids And Bases vrogue.co Best Indicator For Strong Base And Weak Acid Weak acid v strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. In the titration of a weak acid with a strong base, which indicator would be the best choice? Here is a chart of common ph indicators, their ph range, their solutions,. Any of the three. Best Indicator For Strong Base And Weak Acid.
From mmerevise.co.uk
pH Curves Questions and Revision MME Best Indicator For Strong Base And Weak Acid However, the phenolphthalein changes colour exactly where you want it to. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Weak acid v strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Phenolphthalein is usually preferred because of its more easily seen colour. Best Indicator For Strong Base And Weak Acid.
From guitarscalechart.z28.web.core.windows.net
acid and base ph scale chart Show me the ph scale Best Indicator For Strong Base And Weak Acid The correct answer is c. However, the phenolphthalein changes colour exactly where you want it to. Weak acid v strong base. Here is a chart of common ph indicators, their ph range, their solutions,. This time, the methyl orange is hopeless! Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, which. Best Indicator For Strong Base And Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. This time, the methyl orange is hopeless! When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Phenolphthalein is usually preferred because of its more easily seen colour change. However, the phenolphthalein changes colour exactly where you. Best Indicator For Strong Base And Weak Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids Best Indicator For Strong Base And Weak Acid In the titration of a weak acid with a strong base, which indicator would be the best choice? The reaction of the weak acid, acetic acid, with a strong base, naoh,. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Phenolphthalein is usually preferred because of its more easily. Best Indicator For Strong Base And Weak Acid.
From slideplayer.com
Acids and Bases Chapter ppt download Best Indicator For Strong Base And Weak Acid This time, the methyl orange is hopeless! Weak acid v strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. Here is a chart of common ph indicators, their ph range, their solutions,. The correct answer is c. In the titration of a weak acid with a strong base, which indicator would be the best choice?. Best Indicator For Strong Base And Weak Acid.
From www.sexiezpicz.com
Ppt Acid Base Universal Indicator And Ph Scale Powerpoint SexiezPicz Best Indicator For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Any of the three indicators will exhibit. Weak acid v strong base. However, the phenolphthalein changes. Best Indicator For Strong Base And Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Best Indicator For Strong Base And Weak Acid However, the phenolphthalein changes colour exactly where you want it to. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit. Here is a chart of common ph indicators, their ph range, their solutions,. Weak acid v strong base.. Best Indicator For Strong Base And Weak Acid.
From www.oceanproperty.co.th
What Are Some Examples Of Strong And Weak Acids And Bases?, 54 OFF Best Indicator For Strong Base And Weak Acid In the titration of a weak acid with a strong base, which indicator would be the best choice? The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The correct answer is c. However, the phenolphthalein changes colour exactly where you want it to. When titrating. Best Indicator For Strong Base And Weak Acid.
From ecampusontario.pressbooks.pub
17.7 Relative Strengths of Acids and Bases Enhanced Introductory Best Indicator For Strong Base And Weak Acid Weak acid v strong base. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the. Best Indicator For Strong Base And Weak Acid.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Best Indicator For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. Weak acid v strong base. This time, the methyl orange is hopeless! The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base,. Best Indicator For Strong Base And Weak Acid.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Best Indicator For Strong Base And Weak Acid In the titration of a weak acid with a strong base, which indicator would be the best choice? Here is a chart of common ph indicators, their ph range, their solutions,. The reaction of the weak acid, acetic acid, with a strong base, naoh,. This time, the methyl orange is hopeless! The correct answer is c. However, the phenolphthalein changes. Best Indicator For Strong Base And Weak Acid.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. Phenolphthalein is usually preferred because of its more easily seen colour change. Weak acid v strong base. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The titration of. Best Indicator For Strong Base And Weak Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Phenolphthalein is usually. Best Indicator For Strong Base And Weak Acid.
From ar.inspiredpencil.com
Acids And Bases List Best Indicator For Strong Base And Weak Acid This time, the methyl orange is hopeless! In the titration of a weak acid with a strong base, which indicator would be the best choice? Here is a chart of common ph indicators, their ph range, their solutions,. Weak acid v strong base. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The titration of a. Best Indicator For Strong Base And Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Best Indicator For Strong Base And Weak Acid When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Weak acid v strong base. Here is a chart of common ph indicators, their ph range, their solutions,. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. Best Indicator For Strong Base And Weak Acid.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Best Indicator For Strong Base And Weak Acid This time, the methyl orange is hopeless! However, the phenolphthalein changes colour exactly where you want it to. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Phenolphthalein is usually preferred because of its more easily seen colour change. In the titration of a weak acid with a strong base, which indicator would be the best. Best Indicator For Strong Base And Weak Acid.
From www.shimiyar.com
اسید قوی چیست و چه تفاوتی با اسید ضعیف دارد؟ شیمی یار Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. Any of the three indicators will exhibit. Weak acid v strong base. However, the phenolphthalein changes colour exactly where you want it to. Here is a chart of common ph indicators, their ph range, their solutions,. Phenolphthalein is usually preferred because of its more easily seen colour. Best Indicator For Strong Base And Weak Acid.
From webmis.highland.cc.il.us
AcidBase Titrations Best Indicator For Strong Base And Weak Acid Here is a chart of common ph indicators, their ph range, their solutions,. In the titration of a weak acid with a strong base, which indicator would be the best choice? This time, the methyl orange is hopeless! Phenolphthalein is usually preferred because of its more easily seen colour change. However, the phenolphthalein changes colour exactly where you want it. Best Indicator For Strong Base And Weak Acid.
From study.com
Acid & Base Properties of Water Overview & pH Measurement Lesson Best Indicator For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. In the titration of a weak acid with a strong base, which indicator would be the best choice? The reaction of the weak acid, acetic acid, with a strong base, naoh,. The titration of a weak acid with a strong base involves the direct transfer of protons from. Best Indicator For Strong Base And Weak Acid.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Atoms First Best Indicator For Strong Base And Weak Acid Here is a chart of common ph indicators, their ph range, their solutions,. Phenolphthalein is usually preferred because of its more easily seen colour change. This time, the methyl orange is hopeless! Weak acid v strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Any of the. Best Indicator For Strong Base And Weak Acid.
From chem.libretexts.org
16.5 Strong Acids and Bases Chemistry LibreTexts Best Indicator For Strong Base And Weak Acid When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. This time, the methyl orange is hopeless! Weak acid v strong base. However, the phenolphthalein changes colour exactly where you want it to. In the titration of a weak acid with a strong base, which indicator would be the best. Best Indicator For Strong Base And Weak Acid.
From www.ck12.org
Acids and Bases CK12 Foundation Best Indicator For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. In the titration of a weak acid with a strong base, which indicator would be the best choice? Weak acid v strong base. Here is a chart of common ph indicators, their ph range, their solutions,. Any of the three indicators will exhibit. Phenolphthalein is usually preferred. Best Indicator For Strong Base And Weak Acid.
From www.pinterest.com
Strong Acids and Bases MCAT Chemistry Cheat Sheet Study Guide StudyPK Best Indicator For Strong Base And Weak Acid Here is a chart of common ph indicators, their ph range, their solutions,. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Phenolphthalein is usually preferred because of its more easily seen colour change. Weak acid v strong base. Any of the three indicators will exhibit. When titrating strong acids or bases, aim for a ph. Best Indicator For Strong Base And Weak Acid.
From www.sigmaaldrich.com
Acid and Base Chart — Table of Acids & Bases Best Indicator For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. However, the phenolphthalein changes colour exactly where you want it to. The correct answer is c. This time, the methyl orange is hopeless! The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. When. Best Indicator For Strong Base And Weak Acid.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Best Indicator For Strong Base And Weak Acid However, the phenolphthalein changes colour exactly where you want it to. Phenolphthalein is usually preferred because of its more easily seen colour change. Any of the three indicators will exhibit. Here is a chart of common ph indicators, their ph range, their solutions,. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Weak acid v strong. Best Indicator For Strong Base And Weak Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Best Indicator For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. The correct answer is c. However, the phenolphthalein changes colour exactly where you want it to. In the titration of a weak acid with a strong base, which indicator would be the best choice? Here is a chart of common ph indicators, their ph range, their solutions,. Weak. Best Indicator For Strong Base And Weak Acid.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Best Indicator For Strong Base And Weak Acid This time, the methyl orange is hopeless! The reaction of the weak acid, acetic acid, with a strong base, naoh,. The correct answer is c. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Weak acid v strong base. Any of the three indicators will exhibit. In the titration. Best Indicator For Strong Base And Weak Acid.