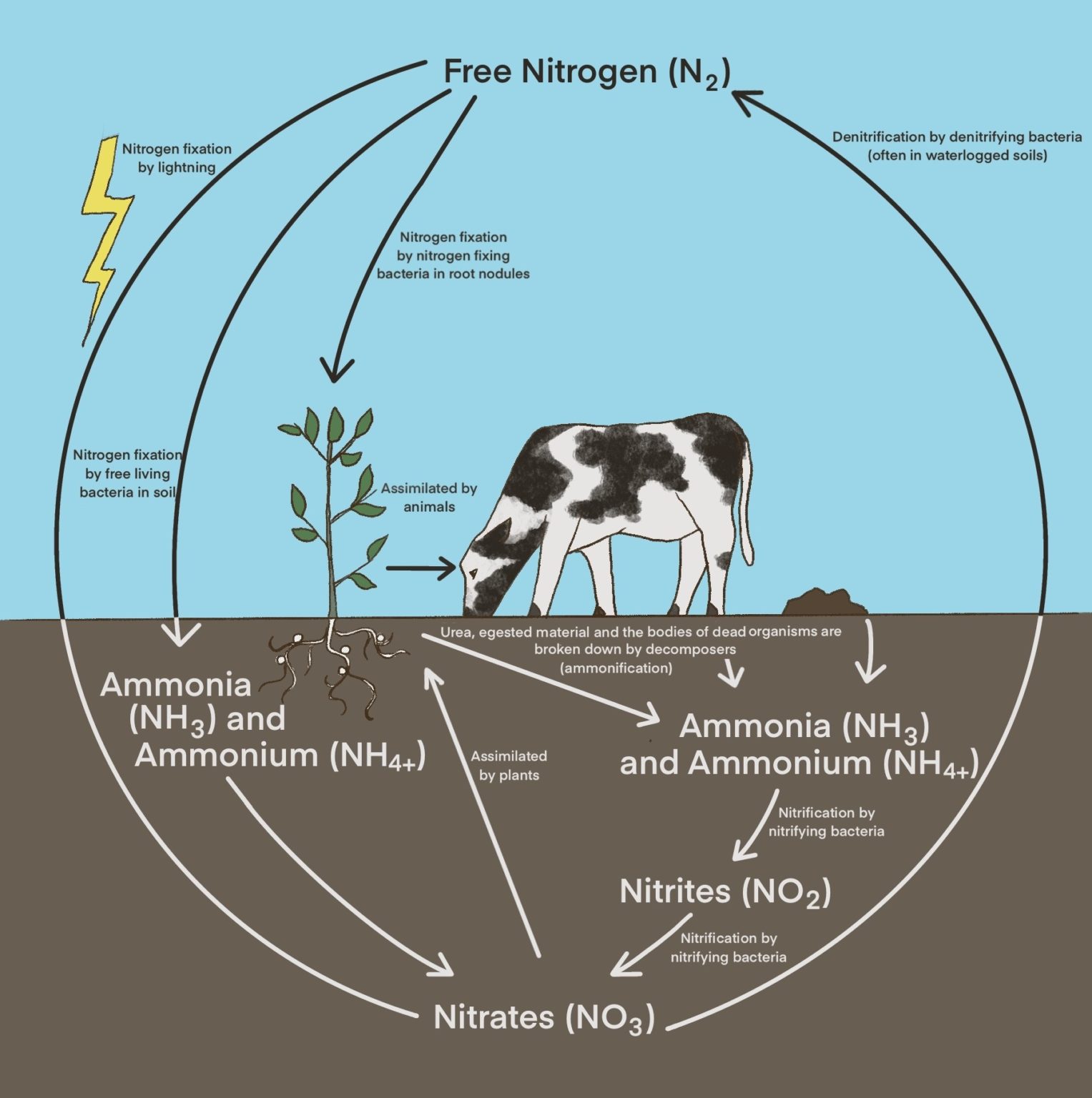
What Is Nitrogen Fixation For Class 9 . Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). In the soil, nitric acid gets. On the basis of agents through which. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation.
from qcebiologyrevision.com
In the soil, nitric acid gets. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. On the basis of agents through which.
Nitrogen Cycle QCE Biology Revision
What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). On the basis of agents through which. In the soil, nitric acid gets. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation.
From byjus.com
What does cyanobacteria do in nitrogen fixation? What Is Nitrogen Fixation For Class 9 To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. In. What Is Nitrogen Fixation For Class 9.
From www.tutoroot.com
What is Nitrogen Cycle? Diagram, Stages, Importance Tutoroot What Is Nitrogen Fixation For Class 9 The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. To explain nitrogen fixation, it is one of the early. What Is Nitrogen Fixation For Class 9.
From www.britannica.com
Understanding the mechanism of nitrogen fixation Britannica What Is Nitrogen Fixation For Class 9 The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Nitrogen fixation converts or ‘fixes’ nitrogen into a form. What Is Nitrogen Fixation For Class 9.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn What Is Nitrogen Fixation For Class 9 Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. On the basis of agents through which. In the soil,. What Is Nitrogen Fixation For Class 9.
From qcebiologyrevision.com
Nitrogen Cycle QCE Biology Revision What Is Nitrogen Fixation For Class 9 On the basis of agents through which. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh. What Is Nitrogen Fixation For Class 9.
From www.sciencefacts.net
Nitrogen Fixation Definition, Process, Example & Equation What Is Nitrogen Fixation For Class 9 On the basis of agents through which. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). It is the conversion of atmospheric nitrogen (n 2) into ammonia. What Is Nitrogen Fixation For Class 9.
From www.priyamstudycentre.com
Nitrogen Fixation Definition, Process, Examples What Is Nitrogen Fixation For Class 9 It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. On the basis of agents through which. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which. What Is Nitrogen Fixation For Class 9.
From www.carmeninthegarden.com
How Nitrogen Fixation Works in Your Garden Carmen in the Garden What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Energy from lightning. What Is Nitrogen Fixation For Class 9.
From ar.inspiredpencil.com
Nitrogen Fixation Diagram What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. Nitrogen fixation converts or. What Is Nitrogen Fixation For Class 9.
From www.sciencefacts.net
Nitrogen Cycle Definition, Steps, Importance with Diagram What Is Nitrogen Fixation For Class 9 It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. On the basis of agents through which. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable. What Is Nitrogen Fixation For Class 9.
From www.adda247.com
Nitrogen Cycle Diagram, Steps, Drawing for Class 8 & 9 What Is Nitrogen Fixation For Class 9 Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). On the basis of agents through which. In the soil, nitric acid. What Is Nitrogen Fixation For Class 9.
From www.geeksforgeeks.org
Nitrogen Fixation Definition, Types, Properties, Examples, FAQs What Is Nitrogen Fixation For Class 9 On the basis of agents through which. In the soil, nitric acid gets. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). The phenomenon. What Is Nitrogen Fixation For Class 9.
From gamesmartz.com
Nitrogen Fixation Definition & Image GameSmartz What Is Nitrogen Fixation For Class 9 To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is. What Is Nitrogen Fixation For Class 9.
From ruralboss.com.au
Nitrogen microbial mineralization & fixation What Is Nitrogen Fixation For Class 9 The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. On the basis of agents through which. It is. What Is Nitrogen Fixation For Class 9.
From phycoterra.com
The Importance of NFixation and Inoculation PhycoTerra® What Is Nitrogen Fixation For Class 9 Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. In the soil, nitric acid gets. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. On the basis of agents through which. Nitrogen fixation, any natural. What Is Nitrogen Fixation For Class 9.
From ar.inspiredpencil.com
Nitrogen Fixation Diagram For Kids What Is Nitrogen Fixation For Class 9 On the basis of agents through which. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia).. What Is Nitrogen Fixation For Class 9.
From sciencenotes.org
Nitrogen Fixation Definition and Processes What Is Nitrogen Fixation For Class 9 Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. In the soil, nitric acid gets. On the basis of agents through which. The process of conversion of atmospheric. What Is Nitrogen Fixation For Class 9.
From www.biochemithon.in
Role of Nitrogen in Crops BioChemiThon BioChemiThon What Is Nitrogen Fixation For Class 9 Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The process of. What Is Nitrogen Fixation For Class 9.
From farmpep.net
Biological Nitrogen Fixation What Is Nitrogen Fixation For Class 9 The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial. What Is Nitrogen Fixation For Class 9.
From unacademy.com
What is nitrogen cycle By Unacademy What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion. What Is Nitrogen Fixation For Class 9.
From balkanecologyproject.blogspot.bg
Balkan Ecology Project Nitrogen Fixing Plants What Is Nitrogen Fixation For Class 9 Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. On the basis of agents through. What Is Nitrogen Fixation For Class 9.
From guidelibunveracity.z21.web.core.windows.net
Nitrogen Cycle Diagram For Class 9 What Is Nitrogen Fixation For Class 9 On the basis of agents through which. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). Energy from lightning breaks. What Is Nitrogen Fixation For Class 9.
From ar.inspiredpencil.com
Nitrogen Cycle Diagram For Class 8 What Is Nitrogen Fixation For Class 9 Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). It is the conversion of atmospheric. What Is Nitrogen Fixation For Class 9.
From ar.inspiredpencil.com
Nitrogen Fixation Definition What Is Nitrogen Fixation For Class 9 Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to. What Is Nitrogen Fixation For Class 9.
From scienceinfo.com
Nitrogen Fixation Definition, Methods, and Benefits What Is Nitrogen Fixation For Class 9 The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants,. What Is Nitrogen Fixation For Class 9.
From www.vedantu.com
What is meant by nitrogen fixation? Explain biological nitrogen What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. On the basis of agents through which. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. Energy from lightning breaks atmospheric molecular. What Is Nitrogen Fixation For Class 9.
From jairku-reilly.blogspot.com
Describe the Process of Nitrogen Fixation JairKuReilly What Is Nitrogen Fixation For Class 9 In the soil, nitric acid gets. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. Nitrogen fixation converts or. What Is Nitrogen Fixation For Class 9.
From www.pinterest.ph
Nitrogen Fixation, Nitrogen Cycle, Class 8, Study Tips, School Projects What Is Nitrogen Fixation For Class 9 To explain nitrogen fixation, it is one of the early stages in the nitrogen cycle, and the conversion takes place using bacterial species such as. On the basis of agents through which. In the soil, nitric acid gets. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. The phenomenon of conversion of free. What Is Nitrogen Fixation For Class 9.
From www.toppr.com
The given diagram shows a part of the nitrogen cycle. Which of the What Is Nitrogen Fixation For Class 9 Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The process of conversion of. What Is Nitrogen Fixation For Class 9.
From www.earth.com
Why is the Nitrogen Cycle So Important? What Is Nitrogen Fixation For Class 9 The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful. What Is Nitrogen Fixation For Class 9.
From www.cell.com
Micromanaging the nitrogen cycle in agroecosystems Trends in Microbiology What Is Nitrogen Fixation For Class 9 Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other elements to form more. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. Energy from lightning breaks atmospheric molecular nitrogen into nitrous. What Is Nitrogen Fixation For Class 9.
From thetreecenter.com
What is Nitrogen Fixation The Tree Center™ What Is Nitrogen Fixation For Class 9 Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by plants, that is ammonia). The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is. What Is Nitrogen Fixation For Class 9.
From sciencenotes.org
Nitrogen Cycle What Is Nitrogen Fixation For Class 9 It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. On the basis of agents through which. Energy from lightning breaks atmospheric molecular nitrogen into nitrous oxide, which reacts with water to form nitrous acid or nitric acid. The process of conversion of atmospheric inert nitrogen gas to fixed nitrogen (inorganic compounds usable by. What Is Nitrogen Fixation For Class 9.
From www.aplustopper.com
What is Nitrogen Fixation and Nitrogen Cycle A Plus Topper What Is Nitrogen Fixation For Class 9 It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation. The process of conversion of atmospheric inert nitrogen gas to. What Is Nitrogen Fixation For Class 9.
From quizlet.com
Carbon & Nitrogen Cycles (12D) Diagram Quizlet What Is Nitrogen Fixation For Class 9 It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. On the basis of agents through which. Nitrogen fixation, any natural or industrial process that causes free nitrogen, which is a relatively inert gas plentiful in air, to combine chemically with other. What Is Nitrogen Fixation For Class 9.