Titration With Hcl And Naoh . In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Hcl + naoh → nacl + h 2 o. Titrate with naoh solution till the first color change. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Naoh + hcl = nacl + h 2 o. Relating titrations to stoichiometry and limiting reagent problems. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. When the acid and base react, they form nacl. Multiply the molarity of the strong base naoh by the volume of. The initial volume and final volume of all three trails were subtracted to find each amount of. Hydrochloric acid reacts with sodium hydroxide. Volume measurements play a key role in titration. According to the reaction equation. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as:
from www.slideserve.com
Hydrochloric acid reacts with sodium hydroxide. Volume measurements play a key role in titration. Hcl + naoh → nacl + h 2 o. When the acid and base react, they form nacl. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titrate with naoh solution till the first color change. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: According to the reaction equation. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that.
PPT Acid Base Titrations PowerPoint Presentation, free download
Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. Titrate with naoh solution till the first color change. According to the reaction equation. Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Hcl + naoh → nacl + h 2 o. Relating titrations to stoichiometry and limiting reagent problems. When the acid and base react, they form nacl. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Volume measurements play a key role in titration. Naoh + hcl = nacl + h 2 o. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide).
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration With Hcl And Naoh According to the reaction equation. Hcl + naoh → nacl + h 2 o. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Volume measurements play a key role. Titration With Hcl And Naoh.
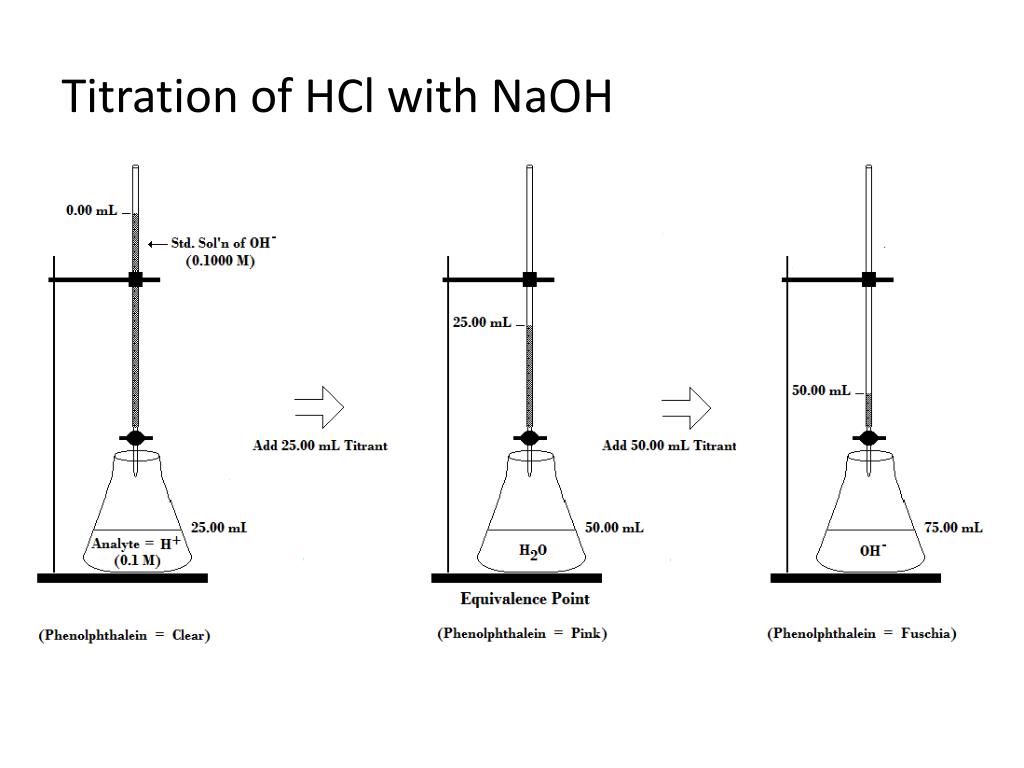
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration With Hcl And Naoh Hydrochloric acid reacts with sodium hydroxide. According to the reaction equation. Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Hcl + naoh → nacl + h. Titration With Hcl And Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration With Hcl And Naoh Hydrochloric acid reacts with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Relating titrations to stoichiometry and limiting reagent problems. Hcl + naoh → nacl + h 2 o. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the. Titration With Hcl And Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration With Hcl And Naoh According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Volume measurements play a key role in titration. Naoh + hcl = nacl + h 2 o. In equation 1, the acid is. Titration With Hcl And Naoh.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Titration With Hcl And Naoh Hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: According to the reaction equation. Naoh + hcl = nacl + h 2 o. Hcl and. Titration With Hcl And Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration With Hcl And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Relating titrations to stoichiometry and limiting reagent problems. Hydrochloric acid reacts with sodium hydroxide. Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh are strong acid and strong. Titration With Hcl And Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration With Hcl And Naoh Multiply the molarity of the strong base naoh by the volume of. The initial volume and final volume of all three trails were subtracted to find each amount of. Titrate with naoh solution till the first color change. Relating titrations to stoichiometry and limiting reagent problems. Volume measurements play a key role in titration. Use this class practical to explore. Titration With Hcl And Naoh.
From www.slideserve.com
PPT Acidity Constant by pH Titration Curves PowerPoint Presentation Titration With Hcl And Naoh In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). According to the reaction equation. Naoh + hcl = nacl + h 2 o. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that.. Titration With Hcl And Naoh.
From mungfali.com
Acid Base Titration Calculation Titration With Hcl And Naoh Titrate with naoh solution till the first color change. Volume measurements play a key role in titration. Hcl + naoh → nacl + h 2 o. Relating titrations to stoichiometry and limiting reagent problems. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react, they form. Titration With Hcl And Naoh.
From www.slideserve.com
PPT Titration Expt. Overview PowerPoint Presentation, free download Titration With Hcl And Naoh Hcl + naoh → nacl + h 2 o. Volume measurements play a key role in titration. Naoh + hcl = nacl + h 2 o. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Relating titrations to stoichiometry. Titration With Hcl And Naoh.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration With Hcl And Naoh Volume measurements play a key role in titration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide. Naoh + hcl = nacl + h 2 o. Hcl + naoh → nacl + h 2 o. When the acid and base react, they form nacl. Hcl. Titration With Hcl And Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration With Hcl And Naoh Multiply the molarity of the strong base naoh by the volume of. The initial volume and final volume of all three trails were subtracted to find each amount of. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. According to the reaction equation. Volume measurements play a. Titration With Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration With Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Naoh + hcl = nacl + h 2 o. According to the reaction equation. Hcl + naoh → nacl + h 2 o. Relating titrations to stoichiometry and limiting reagent problems. Titrate with naoh solution till the first. Titration With Hcl And Naoh.
From mungfali.com
Titration Curve HCl And NaOH Titration With Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. Multiply the molarity of the strong base naoh by the volume of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Titrate with naoh solution till the first color change. According. Titration With Hcl And Naoh.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Titration With Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. When the acid and base react, they form nacl. Relating titrations to stoichiometry and limiting reagent problems. Titrate with naoh solution till the first color change. Naoh + hcl = nacl + h 2 o.. Titration With Hcl And Naoh.
From www.alamy.com
Titration with HCl being added to NaOH and phenolphthalein. First in a Titration With Hcl And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. The initial volume and final volume of all three trails were subtracted to find each amount of. Multiply the molarity of the strong base naoh by the volume of. When the acid and base react, they form nacl. Hcl + naoh → nacl + h 2 o. Relating titrations to stoichiometry and. Titration With Hcl And Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration With Hcl And Naoh Multiply the molarity of the strong base naoh by the volume of. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When the acid and base react, they form nacl. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as:. Titration With Hcl And Naoh.
From theedge.com.hk
Chemistry How To Titration The Edge Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. When the acid and base react, they form nacl. Hydrochloric acid reacts with sodium hydroxide. Naoh + hcl = nacl + h 2 o. According to the reaction equation. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. In. Titration With Hcl And Naoh.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration With Hcl And Naoh Naoh + hcl = nacl + h 2 o. Multiply the molarity of the strong base naoh by the volume of. Relating titrations to stoichiometry and limiting reagent problems. Volume measurements play a key role in titration. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). The initial volume and final volume of. Titration With Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration With Hcl And Naoh Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Titrate. Titration With Hcl And Naoh.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. When the acid and base react, they form nacl. Hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. Titrate with naoh solution till the first color change. Use this class. Titration With Hcl And Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. Hcl + naoh → nacl +. Titration With Hcl And Naoh.
From saylordotorg.github.io
AcidBase Titrations Titration With Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. Multiply the molarity of the strong base naoh by the volume of. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hcl + naoh → nacl + h 2 o. Naoh + hcl =. Titration With Hcl And Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Titration With Hcl And Naoh Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Relating titrations to stoichiometry and limiting reagent problems. When the acid and base react, they form nacl. Volume measurements play a key role in titration. Hcl + naoh → nacl + h 2 o. Consider the titration of \(\ce{hcl}\). Titration With Hcl And Naoh.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration With Hcl And Naoh Hcl + naoh → nacl + h 2 o. According to the reaction equation. Titrate with naoh solution till the first color change. When the acid and base react, they form nacl. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. Relating titrations to. Titration With Hcl And Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration With Hcl And Naoh Titrate with naoh solution till the first color change. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Hydrochloric acid reacts with sodium hydroxide. Volume measurements play a key role in. Titration With Hcl And Naoh.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Titration With Hcl And Naoh Multiply the molarity of the strong base naoh by the volume of. Naoh + hcl = nacl + h 2 o. The initial volume and final volume of all three trails were subtracted to find each amount of. Hydrochloric acid reacts with sodium hydroxide. When the acid and base react, they form nacl. Since hcl and naoh fully dissociate into. Titration With Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration With Hcl And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Relating titrations to stoichiometry and limiting reagent problems. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Volume measurements play a key role in titration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride. Titration With Hcl And Naoh.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration With Hcl And Naoh Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Naoh + hcl = nacl + h 2 o. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Relating titrations to stoichiometry and limiting reagent problems.. Titration With Hcl And Naoh.
From saylordotorg.github.io
AcidBase Titrations Titration With Hcl And Naoh In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Titrate with naoh solution till the first color change. Volume measurements play a key role in titration. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Hydrochloric acid reacts with sodium. Titration With Hcl And Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration With Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Hcl + naoh → nacl + h 2 o. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium. Titration With Hcl And Naoh.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration With Hcl And Naoh Volume measurements play a key role in titration. The initial volume and final volume of all three trails were subtracted to find each amount of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Naoh + hcl = nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide. When the acid and base react, they form nacl. Multiply the molarity. Titration With Hcl And Naoh.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration With Hcl And Naoh Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Titrate with naoh solution till the first color change. According to the reaction equation. Hcl + naoh → nacl + h 2 o. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and. Titration With Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. Volume measurements play a key role in titration. In equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that.. Titration With Hcl And Naoh.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration With Hcl And Naoh Relating titrations to stoichiometry and limiting reagent problems. The initial volume and final volume of all three trails were subtracted to find each amount of. Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that. Hydrochloric acid reacts with sodium hydroxide. In equation 1, the acid is hcl (hydrochloric acid) and the base. Titration With Hcl And Naoh.