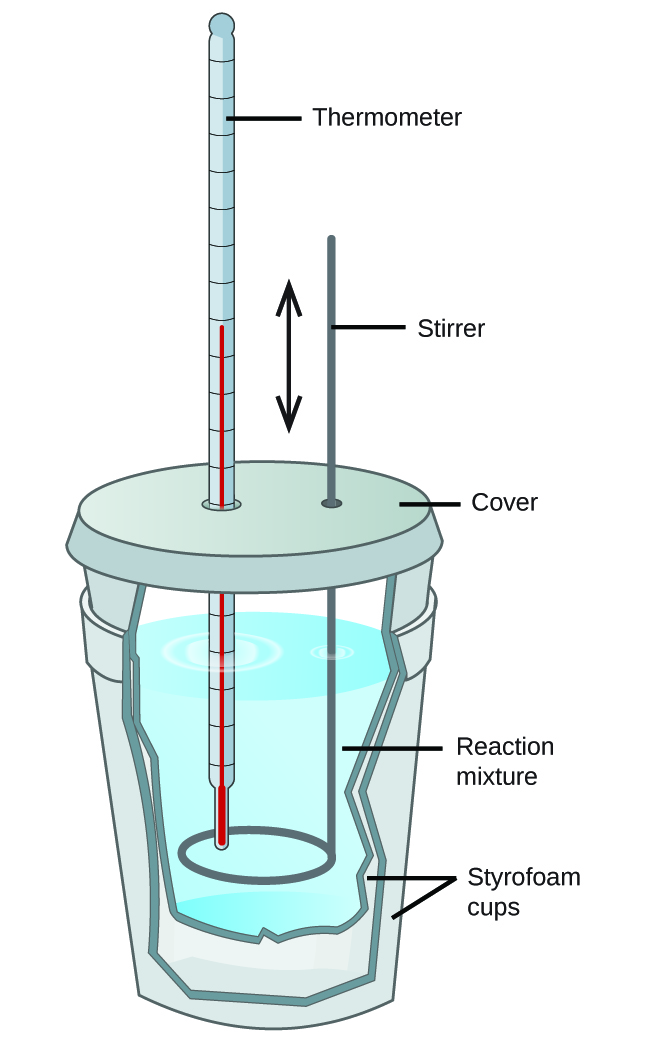
Calorimeter What Mean . Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.
from tukioka-clinic.com
Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What
Calorimeter What Mean Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter What Mean A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is a branch. Calorimeter What Mean.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter What Mean Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. To. Calorimeter What Mean.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter What Mean The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A calorimeter is an instrument that has a thermally isolated. Calorimeter What Mean.
From pixelrz.com
Calorimeter Room Calorimeter What Mean Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. To determine the enthalpy, stability, heat capacity, and other thermochemical. Calorimeter What Mean.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter What Mean Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry. Calorimeter What Mean.
From saylordotorg.github.io
Calorimetry Calorimeter What Mean Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat flows in and out of. Calorimeter What Mean.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimeter What Mean A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimeter What Mean.
From www.animalia-life.club
Calorimeter Diagram Calorimeter What Mean Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The amount of heat released or absorbed during a reaction is determined by measuring. Calorimeter What Mean.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Calorimeter What Mean Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeters are used to measure the heat of combustion, heat capacity, and. Calorimeter What Mean.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Calorimeter What Mean Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeters are used to measure the heat of. Calorimeter What Mean.
From byjus.com
What is bomb calorimeter? Calorimeter What Mean Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction.. Calorimeter What Mean.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter What Mean A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using. Calorimeter What Mean.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter What Mean A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the measurement of the. Calorimeter What Mean.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download Calorimeter What Mean Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A. Calorimeter What Mean.
From www.youtube.com
050 Calorimetry YouTube Calorimeter What Mean Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To determine the enthalpy, stability, heat capacity,. Calorimeter What Mean.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Calorimeter What Mean.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Calorimeter What Mean A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeter, device for measuring the heat developed during a. Calorimeter What Mean.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Calorimeter What Mean To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features. Calorimeter What Mean.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of. Calorimeter What Mean.
From testbook.com
Understanding Bomb Calorimeter Working, Construction, and Uses Calorimeter What Mean The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimeter What Mean.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter What Mean Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry. Calorimeter What Mean.
From www.animalia-life.club
Calorimeter Diagram Calorimeter What Mean Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. The amount of heat released or absorbed. Calorimeter What Mean.
From glossary.periodni.com
Download calorimeter.png image from Calorimeter What Mean Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction.. Calorimeter What Mean.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To determine the enthalpy,. Calorimeter What Mean.
From www.animalia-life.club
Calorimeter Diagram Calorimeter What Mean To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A calorimeter. Calorimeter What Mean.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter What Mean Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimeter What Mean.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Calorimeter What Mean Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy. Calorimeter What Mean.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Calorimeter What Mean Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the measurement of the. Calorimeter What Mean.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Calorimeter What Mean Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The amount of heat. Calorimeter What Mean.
From byjus.com
The calorimeter is commonly made up of……..because it has……. Calorimeter What Mean Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Technically,. Calorimeter What Mean.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. Calorimeter What Mean.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet Calorimeter What Mean Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a. Calorimeter What Mean.
From manueldesnhray.blogspot.com
Identify What a Coffee Cup Calorimeter Measures. Calorimeter What Mean Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in. Calorimeter What Mean.
From socratic.org
A student conducting a calorimetry investigation determines a negative Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Technically, this is called an adiabatic. Calorimeter What Mean.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter What Mean Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically,. Calorimeter What Mean.