Titration Balanced Equation . Titration between nitric acid and sodium carbonate is represented by the equation. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a titration to see how much acid. When an acid and alkali react together, a neutralisation reaction occurs.
from www.numerade.com
A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Titration between nitric acid and sodium carbonate is represented by the equation. Here, we will consider titrations that involve acid. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Writing a balanced chemical equation. When an acid and alkali react together, a neutralisation reaction occurs. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. If we have an unknown concentration of acid, we can do a titration to see how much acid.
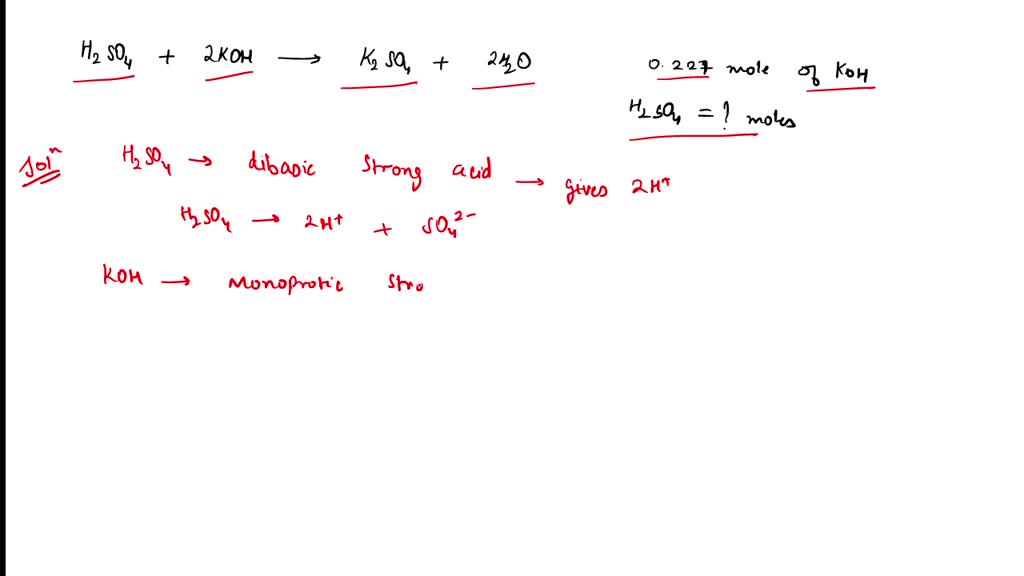
A student carried out a titration using H2SO4 and KOH. The balanced
Titration Balanced Equation Titration between nitric acid and sodium carbonate is represented by the equation. Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. If we have an unknown concentration of acid, we can do a titration to see how much acid. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. When an acid and alkali react together, a neutralisation reaction occurs.
From adelaideewawood.blogspot.com
H2so4 Naoh Balanced Equation AdelaideewaWood Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is. Titration Balanced Equation.
From www.chegg.com
Solved Write a balanced equation for the reaction of sodium Titration Balanced Equation Here, we will consider titrations that involve acid. Titration between nitric acid and sodium carbonate is represented by the equation. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. If we have an unknown. Titration Balanced Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. If we have an unknown concentration of acid, we can do a titration to see how much acid. Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider. Titration Balanced Equation.
From www.numerade.com
SOLVED Write the equation for the titration of Ca2+ with EDTA. Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a titration to see how much acid. 2h. Titration Balanced Equation.
From hxesplqrv.blob.core.windows.net
Titration Balanced Equation Formula at Brandon Palmer blog Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n. Titration Balanced Equation.
From www.numerade.com
SOLVED A sample of ascorbic acid, with the formula C6H8O6, is to be Titration Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. When an acid and. Titration Balanced Equation.
From shotprofessional22.gitlab.io
Ace Khp And Naoh Balanced Equation Physics Wallah 12th Class Chemistry Pdf Titration Balanced Equation Writing a balanced chemical equation. Here, we will consider titrations that involve acid. Titration between nitric acid and sodium carbonate is represented by the equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a titration to see how much. Titration Balanced Equation.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube Titration Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Here, we will consider titrations that involve acid. Writing a balanced chemical equation. When an acid and alkali react together, a neutralisation reaction occurs. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. A titration can be performed with almost any chemical reaction for. Titration Balanced Equation.
From www.chegg.com
Solved Consider a different titration for this exercise. Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is. Titration Balanced Equation.
From www.numerade.com
SOLVED 14.Write the equilibrium constant expression for the Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider titrations that involve acid. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium. Titration Balanced Equation.
From ar.inspiredpencil.com
Titration Equation Titration Balanced Equation Writing a balanced chemical equation. When an acid and alkali react together, a neutralisation reaction occurs. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Here, we will consider titrations that involve acid. A titration can be performed with almost any. Titration Balanced Equation.
From www.numerade.com
SOLVED 1. Gianna performed acidbase titration to determine the Titration Balanced Equation Titration between nitric acid and sodium carbonate is represented by the equation. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. If we have an unknown concentration of acid, we can do a titration to see how much acid. Writing a balanced chemical equation.. Titration Balanced Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Balanced Equation When an acid and alkali react together, a neutralisation reaction occurs. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider titrations that involve acid. If we have an unknown concentration of acid, we can do a titration to see how much acid. Naoh (aq) + hcl (aq). Titration Balanced Equation.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Balanced Equation Here, we will consider titrations that involve acid. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. If we have an unknown concentration of acid, we can do a titration to see how much acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. A. Titration Balanced Equation.
From www.numerade.com
SOLVED What is a balanced equation for the titration of Mg2+ with EDTA? Titration Balanced Equation When an acid and alkali react together, a neutralisation reaction occurs. Titration between nitric acid and sodium carbonate is represented by the equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a titration to see how much acid. Writing. Titration Balanced Equation.
From www.numerade.com
SOLVED Oxalic acid dihydrate (H2C2O4 · 2H2O) can be used to Titration Balanced Equation Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. When an acid and alkali react. Titration Balanced Equation.
From www.youtube.com
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate Titration Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium carbonate is represented by the equation. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a. Titration Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Balanced Equation When an acid and alkali react together, a neutralisation reaction occurs. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. The above equation works only for. Titration Balanced Equation.
From ar.inspiredpencil.com
Titration Equation Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. If we have an unknown concentration of acid, we can do a titration to see how much acid. When an acid and alkali react together, a neutralisation reaction occurs. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Writing a balanced. Titration Balanced Equation.
From unacademy.com
Mohr salt titration Titration Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. The above equation works only for neutralizations. Titration Balanced Equation.
From orvelleblog.blogspot.com
Spice of Lyfe Chemical Equation For Khp And Naoh Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which. Titration Balanced Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium carbonate is represented by the equation.. Titration Balanced Equation.
From www.numerade.com
SOLVED what happens when sodium hydroxide reacts with sulphuric acid Titration Balanced Equation Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider titrations that involve acid. If we have an unknown concentration of acid, we can do a titration to see how much acid. When an acid and alkali react together, a neutralisation reaction occurs.. Titration Balanced Equation.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Titration Balanced Equation If we have an unknown concentration of acid, we can do a titration to see how much acid. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Writing a balanced chemical equation. Here, we will consider titrations that involve acid. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be. Titration Balanced Equation.
From ar.inspiredpencil.com
Titration Equation Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Here, we will consider titrations that involve acid. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation. If we have an unknown concentration of acid, we can do a. Titration Balanced Equation.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. When an acid and alkali react together, a neutralisation reaction occurs. Titration between nitric acid and sodium carbonate is represented by the equation. If we have an unknown concentration of acid, we can do a titration to see how much acid. Here,. Titration Balanced Equation.
From boys.velvet.jp
Net Ionic Equation For Na2CO3 HCl Sodium Carbonate, 42 OFF Titration Balanced Equation If we have an unknown concentration of acid, we can do a titration to see how much acid. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Writing a balanced chemical equation. The above equation works only for neutralizations in which. Titration Balanced Equation.
From byjus.com
24.Why their is difference when naoh and na2co3 are titrated with Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we. Titration Balanced Equation.
From mungfali.com
HCl NaOH Titration Titration Balanced Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration between nitric acid and sodium carbonate is represented by the equation. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Here, we will consider titrations that involve acid. A titration can be performed with almost any chemical reaction. Titration Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations, including states of matter Titration Balanced Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. If we have an unknown concentration of acid, we can do a titration to see how much acid. When an acid and alkali react together, a neutralisation reaction occurs. Titration between nitric acid and sodium carbonate is represented by the. Titration Balanced Equation.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID5572517 Titration Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. If we have an unknown concentration of acid, we can do a titration to see how much acid.. Titration Balanced Equation.
From www.vrogue.co
Solved The Balanced Equation For The Titration Of Oxa vrogue.co Titration Balanced Equation If we have an unknown concentration of acid, we can do a titration to see how much acid. Here, we will consider titrations that involve acid. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so. Titration Balanced Equation.
From www.youtube.com
Titration Stoichiometry HCl and Ba(OH)2 YouTube Titration Balanced Equation Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Writing a balanced chemical equation. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider titrations that involve acid. A titration can be. Titration Balanced Equation.
From mungfali.com
Acid Base Titration Calculation Titration Balanced Equation Here, we will consider titrations that involve acid. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation. When an acid and alkali react together, a neutralisation reaction occurs. A titration can be performed with almost any chemical reaction for which the balanced chemical equation. Titration Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of KHP Titration Balanced Equation A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. When an acid and alkali react together, a neutralisation reaction occurs. Here, we will. Titration Balanced Equation.