M/Z In Chemistry . In mass analysis, an electron is taken from molecules. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which this value can be obtained. M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. Its structure is uncertain, but two possibilities are shown in the. Mass/charge ratio is given the symbol m/z (or sometimes m/e). The third strongest ion in the spectrum has m/z=39 (c 3 h 3). An ion with a mass of 56 and a. The mass error of an assignment,. This page allows you to perform three calculations: Mass error and m/z from formula. In mass analysis, an electron is taken from molecules to create single charged ions.
from www.studocu.com
An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which this value can be obtained. This page allows you to perform three calculations: In mass analysis, an electron is taken from molecules to create single charged ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules. M stands for mass and z stands for charge number of ions. M stands for mass and z stands for charge number of ions.
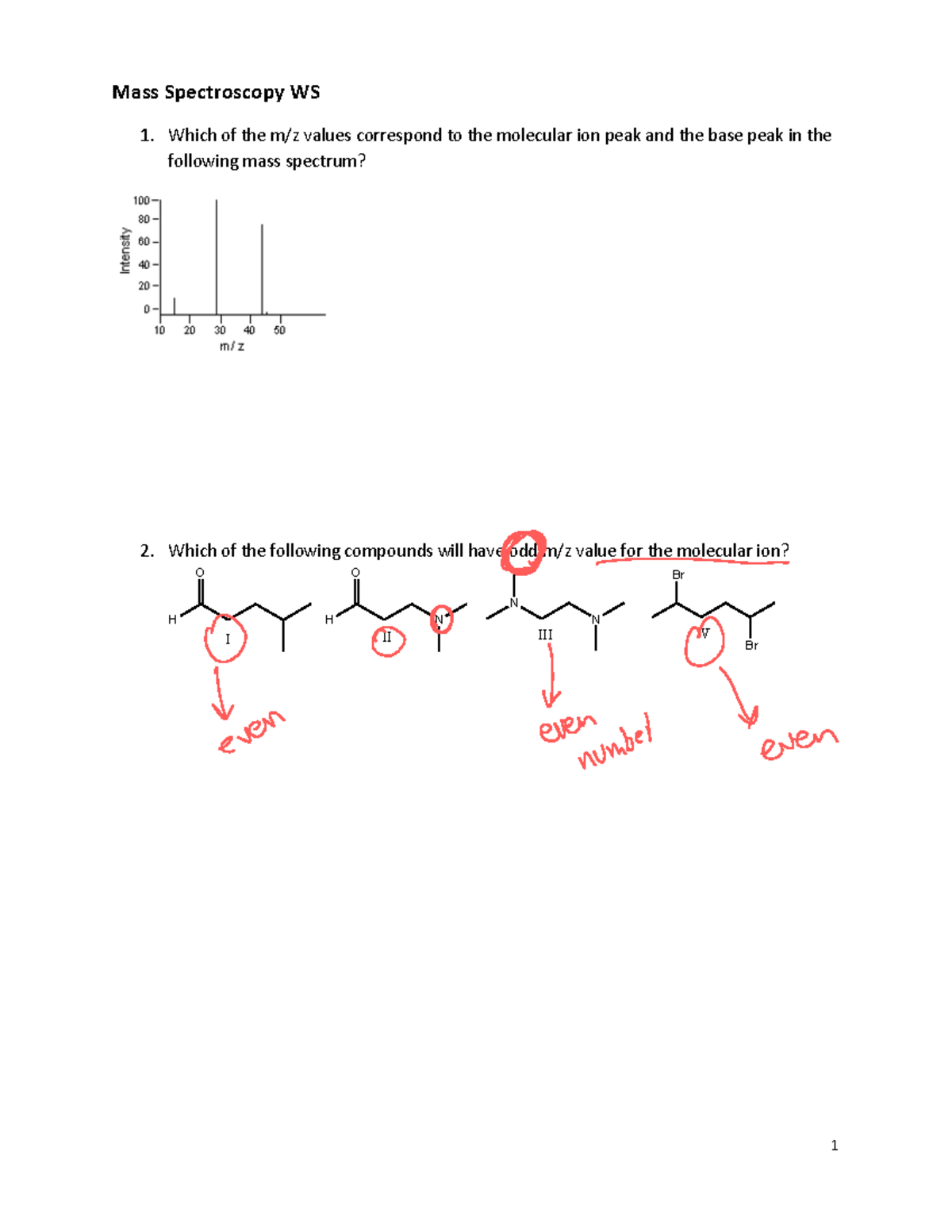
MassSpecWS Mass spec worksheet Mass Spectroscopy WS 1. Which of
M/Z In Chemistry In mass analysis, an electron is taken from molecules. The mass error of an assignment,. Mass/charge ratio is given the symbol m/z (or sometimes m/e). This page allows you to perform three calculations: For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is taken from molecules. In mass analysis, an electron is taken from molecules to create single charged ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Its structure is uncertain, but two possibilities are shown in the. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass error and m/z from formula. An ion with a mass of 56 and a. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which this value can be obtained. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). M stands for mass and z stands for charge number of ions.
From infinitylearn.com
Chemistry Formulas All Chemistry Formula List Infinity Learn M/Z In Chemistry Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. This page allows you to perform three calculations: Suggest possible molecular formulas for a compound,. M/Z In Chemistry.
From www.slideserve.com
PPT Chapter 2. Molecular Weight and Polymer Solutions PowerPoint M/Z In Chemistry An ion with a mass of 56 and a. M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M stands for mass and z stands for. M/Z In Chemistry.
From mayanguyen.com
Periodic Table Of Elements With Names And Symbols Pdf M/Z In Chemistry M stands for mass and z stands for charge number of ions. Mass error and m/z from formula. Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which this value can be obtained. An ion with a mass of 56 and a charge of 2+ would also have a. M/Z In Chemistry.
From www.numerade.com
SOLVED What is the m/z value (to the nearest whole number) of the M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. This page allows you to perform three calculations: In mass analysis, an electron is taken from molecules to create single charged ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a. M/Z In Chemistry.
From mungfali.com
Organic Chemistry Nomenclature Chart M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Its structure is uncertain, but two possibilities are shown in the. This page allows you to perform three calculations: Mass/charge ratio is given the symbol m/z (or sometimes m/e). An ion with a mass of 56 and a. M. M/Z In Chemistry.
From www.masterorganicchemistry.com
Alkene Nomenclature Cis and Trans and E and Z — Master Organic Chemistry M/Z In Chemistry M stands for mass and z stands for charge number of ions. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). In mass analysis, an electron is taken from molecules to create single charged ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). An ion with a mass of 56 and a charge of. M/Z In Chemistry.
From www.conquerhsc.com
HSC Chemistry Module 8 Inquiry Question 2 M/Z In Chemistry Mass error and m/z from formula. In mass analysis, an electron is taken from molecules to create single charged ions. This page allows you to perform three calculations: For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of. M/Z In Chemistry.
From www.chemicool.com
Periodic Table of Elements and Chemistry M/Z In Chemistry Its structure is uncertain, but two possibilities are shown in the. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). M stands for mass and z stands for charge number of ions. In mass analysis, an electron is taken from molecules to create single charged ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e).. M/Z In Chemistry.
From www.researchgate.net
Detailed information regarding observed and theoretical m/z values for M/Z In Chemistry Mass error and m/z from formula. The mass error of an assignment,. In mass analysis, an electron is taken from molecules. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions. An ion with a mass of. M/Z In Chemistry.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent M/Z In Chemistry Its structure is uncertain, but two possibilities are shown in the. Mass/charge ratio is given the symbol m/z (or sometimes m/e). The third strongest ion in the spectrum has m/z=39 (c 3 h 3). An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. Mass error and m/z from formula.. M/Z In Chemistry.
From www.tessshebaylo.com
Mass Spectrometry Equation Chemistry Tessshebaylo M/Z In Chemistry Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass error and m/z from formula. In mass analysis, an electron is taken from molecules. In mass analysis, an electron is taken from molecules to create single charged ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28.. M/Z In Chemistry.
From www.youtube.com
Organic Chemistry Concepts [AZ] in just 1 Hour GOC PLAY Chemistry M/Z In Chemistry The third strongest ion in the spectrum has m/z=39 (c 3 h 3). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Its structure is uncertain, but two possibilities are shown in the. In mass analysis, an electron is taken from molecules. For example, if an ion had. M/Z In Chemistry.
From www.chegg.com
Solved Which compound has a molecular ion at m/z = 58, an IR M/Z In Chemistry An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. In mass analysis, an electron is taken from molecules to create single charged ions. In mass analysis, an electron is taken from molecules. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass error and m/z from formula. For. M/Z In Chemistry.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry M/Z In Chemistry Its structure is uncertain, but two possibilities are shown in the. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. In mass analysis, an electron is taken from molecules. M stands for mass and z stands for charge number of ions. This page allows you to perform three calculations:. M/Z In Chemistry.
From www.chemicals.co.uk
What Is An Element In Chemistry? (5 Examples) The Chemistry Blog M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is taken from molecules to create single charged ions. The mass error of an assignment,. In mass analysis, an electron is taken from molecules. Mass error and m/z from formula. An ion with a. M/Z In Chemistry.
From www.chegg.com
Solved Which of the molecules below would be consistent with M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). This page allows you to perform three calculations: The mass error of an assignment,. M stands for. M/Z In Chemistry.
From www.youtube.com
mass spectrometry even and odd m/z values YouTube M/Z In Chemistry In mass analysis, an electron is taken from molecules to create single charged ions. M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. The mass error of an assignment,. An ion with a mass of 56. M/Z In Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry M M/Z In Chemistry An ion with a mass of 56 and a. In mass analysis, an electron is taken from molecules. This page allows you to perform three calculations: For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M stands for. M/Z In Chemistry.
From www.chegg.com
Solved Identify the m/z corresponding to the base peak and M/Z In Chemistry Mass error and m/z from formula. This page allows you to perform three calculations: Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged ions. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). An ion with a mass of 56 and a.. M/Z In Chemistry.
From www.britannica.com
Elements of the Periodic Table Quiz M/Z In Chemistry Mass/charge ratio is given the symbol m/z (or sometimes m/e). The mass error of an assignment,. In mass analysis, an electron is taken from molecules. An ion with a mass of 56 and a. Its structure is uncertain, but two possibilities are shown in the. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). Mass error. M/Z In Chemistry.
From www.chegg.com
Solved Which of the molecules below would be consistent with M/Z In Chemistry An ion with a mass of 56 and a. M stands for mass and z stands for charge number of ions. Mass error and m/z from formula. In mass analysis, an electron is taken from molecules. In mass analysis, an electron is taken from molecules to create single charged ions. The mass error of an assignment,. Suggest possible molecular formulas. M/Z In Chemistry.
From materialmagicgerste.z13.web.core.windows.net
What Are All The Functional Groups M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Its structure is uncertain, but two possibilities are shown in the. In mass analysis, an electron is taken from molecules. M stands for mass and z stands for charge number of ions. An ion with a mass of 56. M/Z In Chemistry.
From www.chegg.com
Solved The following m/z spectral features were obtained for M/Z In Chemistry M stands for mass and z stands for charge number of ions. Its structure is uncertain, but two possibilities are shown in the. The mass error of an assignment,. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which. M/Z In Chemistry.
From chem.libretexts.org
20.1 Molecular Mass Spectra Chemistry LibreTexts M/Z In Chemistry M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a charge of 2+ would also have. M/Z In Chemistry.
From chem.libretexts.org
B2. Sequence Determination Using Mass Spectrometry Chemistry LibreTexts M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged ions. Mass error and m/z from formula. The mass error of an assignment,. An ion with. M/Z In Chemistry.
From www.researchgate.net
Product ions of m/z 546 mass spectrum of M16 and the proposed origin of M/Z In Chemistry Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). In mass analysis, an electron is. M/Z In Chemistry.
From www.transformationtutoring.com
Percent Abundance and Average Atomic Mass Calculations Guide M/Z In Chemistry In mass analysis, an electron is taken from molecules. In mass analysis, an electron is taken from molecules to create single charged ions. This page allows you to perform three calculations: Its structure is uncertain, but two possibilities are shown in the. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio. M/Z In Chemistry.
From www.researchgate.net
Mass spectra of intermediates with m/z 28, 42, 56, 70, 84, 98, and 112 M/Z In Chemistry Suggest possible molecular formulas for a compound, given the m/z value for the molecular ion, or a mass spectrum from which this value can be obtained. Mass error and m/z from formula. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. This page allows you to perform three. M/Z In Chemistry.
From www.docbrown.info
mass spectrum of 2methylpentane fragmentation pattern of m/z m/e ions M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass error and m/z from formula. Suggest possible molecular formulas for a compound, given the m/z. M/Z In Chemistry.
From www.studocu.com
MassSpecWS Mass spec worksheet Mass Spectroscopy WS 1. Which of M/Z In Chemistry In mass analysis, an electron is taken from molecules to create single charged ions. M stands for mass and z stands for charge number of ions. The third strongest ion in the spectrum has m/z=39 (c 3 h 3). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28.. M/Z In Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating the formula mass from a chemical formula M/Z In Chemistry Mass/charge ratio is given the symbol m/z (or sometimes m/e). An ion with a mass of 56 and a. In mass analysis, an electron is taken from molecules. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Its structure is uncertain, but two possibilities are shown in the. In mass analysis, an electron is taken from molecules to create. M/Z In Chemistry.
From www.researchgate.net
Massto charge ratio, m/z, values of the characteristic product ions of M/Z In Chemistry In mass analysis, an electron is taken from molecules to create single charged ions. An ion with a mass of 56 and a. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass error and m/z from formula. For. M/Z In Chemistry.
From www.scitk.org
Functional Groups in Chemistry M/Z In Chemistry For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass/charge ratio is given the symbol m/z (or. M/Z In Chemistry.
From kunduz.com
[ANSWERED] Use the information given in the diagram to prove that m Z M/Z In Chemistry An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. M stands for mass and z stands for charge number of ions. Mass error and m/z from formula. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules. The third strongest. M/Z In Chemistry.
From www.researchgate.net
Product ions of m/z 546 mass spectrum of M16 and the proposed origin of M/Z In Chemistry An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. This page allows you to perform three calculations: For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. An ion with a mass of 56 and a. For example,. M/Z In Chemistry.