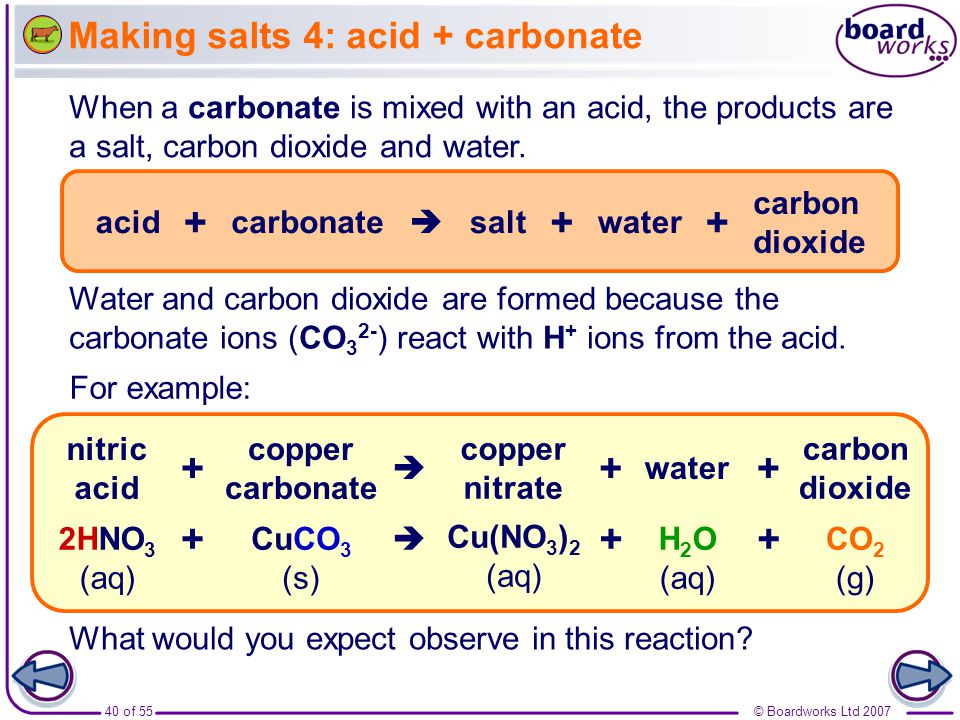
Lead Carbonate + Sulfuric Acid . Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. So a simple filtration will allow the separation lead. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. See examples, equations and videos of the reactions.
from exouyldhc.blob.core.windows.net
So a simple filtration will allow the separation lead. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. See examples, equations and videos of the reactions. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts.
Lead Carbonate + Sulphuric Acid at Harold Petersen blog
Lead Carbonate + Sulfuric Acid So a simple filtration will allow the separation lead. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. See examples, equations and videos of the reactions. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. So a simple filtration will allow the separation lead. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are.
From www.nature-microscope-photo-video.com
Lead carbonate. 01 Covers Photos Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: See examples, equations and videos of the reactions. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. So a simple filtration will allow the separation lead. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions. Lead Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVED The preparation of lead sulphate from lead carbonate is a two Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. So a simple. Lead Carbonate + Sulfuric Acid.
From alchetron.com
Sulfuric acid Alchetron, The Free Social Encyclopedia Lead Carbonate + Sulfuric Acid Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See examples, equations and videos of the reactions. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Learn how. Lead Carbonate + Sulfuric Acid.
From www.westchem.com.au
Sulphuric Acid 33 Industrial Use Westchem Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. Enter an ionic equation and get its complete. Lead Carbonate + Sulfuric Acid.
From biologydictionary.net
Sulfuric Acid The Definitive Guide Biology Dictionary Lead Carbonate + Sulfuric Acid Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. So a simple filtration will allow the separation lead. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and. Lead Carbonate + Sulfuric Acid.
From instrumentationtools.com
How does a Lead Acid Battery Work? Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. Another method for preparing lead sulphate from lead carbonate is by reacting. Lead Carbonate + Sulfuric Acid.
From www.indiamart.com
Lead Carbonate at best price in Vadodara ID 26746766248 Lead Carbonate + Sulfuric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. So a simple filtration will allow the separation lead. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly. Lead Carbonate + Sulfuric Acid.
From www.youtube.com
Lead Ingots & Sulfuric Acid Scavenged From My Car Battery YouTube Lead Carbonate + Sulfuric Acid See examples, equations and videos of the reactions. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. So a simple filtration will allow the. Lead Carbonate + Sulfuric Acid.
From www.indiamart.com
Sulfuric Acid, Liquid at best price in Kadapa ID 22395066433 Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: So a simple filtration will allow the separation lead. Another method for. Lead Carbonate + Sulfuric Acid.
From dir.indiamart.com
Lead Carbonate at Best Price in India Lead Carbonate + Sulfuric Acid Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. See examples, equations and videos of. Lead Carbonate + Sulfuric Acid.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. So a simple filtration will allow the separation lead. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. See examples, equations and videos of the reactions. The chemical equation for the. Lead Carbonate + Sulfuric Acid.
From www.numerade.com
SOLVEDCar Battery Car batteries use lead, lead(IV) oxide, and a Lead Carbonate + Sulfuric Acid So a simple filtration will allow the separation lead. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce. Lead Carbonate + Sulfuric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for HNO3 + Ca(OH)2 YouTube Lead Carbonate + Sulfuric Acid Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. So a simple filtration will allow the separation lead. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a. Lead Carbonate + Sulfuric Acid.
From www.labdepotinc.com
Lead Carbonate, Powder, Reagent, ACS Lead Carbonate + Sulfuric Acid See examples, equations and videos of the reactions. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. The chemical equation for the. Lead Carbonate + Sulfuric Acid.
From www.guidechem.com
CAS 1319466 Lead subcarbonate Manufacturers,suppliers,fob price Lead Carbonate + Sulfuric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. See examples, equations and videos of the reactions. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is:. Lead Carbonate + Sulfuric Acid.
From en.wikipedia.org
FileSulfuricacid3DballsB.png Wikipedia, the free encyclopedia Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead sulfate is. Lead Carbonate + Sulfuric Acid.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. So a simple filtration will allow the separation lead. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When. Lead Carbonate + Sulfuric Acid.
From www.sciencephoto.com
Lithium Carbonate Sulfuric Acid Reaction, 3 of 3 Stock Image C039 Lead Carbonate + Sulfuric Acid Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. So a simple filtration will allow the separation lead. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water. Lead Carbonate + Sulfuric Acid.
From www.indiamart.com
Laboratory Lead Carbonate Liquid at Rs 120/bottle Lead Carbonate in Lead Carbonate + Sulfuric Acid Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See examples, equations and videos of the reactions. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and. Lead Carbonate + Sulfuric Acid.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate + Sulfuric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Lead sulfate is not soluble. Lead Carbonate + Sulfuric Acid.
From www.palangkaset.com
จะหยุด “ กรดซัลฟิวริค ” ต้องแก้จุดอ่อน “ กรดฟอร์มิค ” ยางพารา พลัง Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid. Lead Carbonate + Sulfuric Acid.
From teknonel.com
Car Battery Definition, Types Of Batteries, Inside Parts Teknonel Lead Carbonate + Sulfuric Acid So a simple filtration will allow the separation lead. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: See examples, equations and videos of the reactions. When acids react with carbonates, such as calcium carbonate (found. Lead Carbonate + Sulfuric Acid.
From www.numerade.com
⏩SOLVEDSolutions of sulfuric acid and lead(II) acetate react to Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water. Lead Carbonate + Sulfuric Acid.
From www.askiitians.com
Sulphuric Acid Study Material for IIT JEE askIITians Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: See examples, equations and videos of the reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid. Lead Carbonate + Sulfuric Acid.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are.. Lead Carbonate + Sulfuric Acid.
From www.tradekorea.com
B2B Portal tradekorea, No.1 B2B Marketplace for Korea Manufacturers and Lead Carbonate + Sulfuric Acid See examples, equations and videos of the reactions. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. So a simple filtration will allow the separation lead. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. The chemical equation for the reaction between lead carbonate (pbco3) and. Lead Carbonate + Sulfuric Acid.
From www.ottokemi.com
Lead carbonate, basic 1319466 Manufacturers & Suppliers in India Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: So a simple filtration will allow the separation lead. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. See examples, equations and videos of the reactions. When. Lead Carbonate + Sulfuric Acid.
From www.indiamart.com
Lead Carbonate at best price in Thane by Nithyasri Chemicals ID Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See examples, equations and videos of the reactions. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. When acids react with carbonates, such as. Lead Carbonate + Sulfuric Acid.
From www.exportersindia.com
Lead Carbonate, Purity 99, Packaging Type HDPE Liner Bag at Rs 240 Lead Carbonate + Sulfuric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. See examples, equations and videos of the reactions. So a simple. Lead Carbonate + Sulfuric Acid.
From www.slideserve.com
PPT Na 3 PO 4 + 3 KOH → 3 NaOH + K 3 PO 4 Double Displacement Lead Carbonate + Sulfuric Acid Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See examples, equations and videos of the reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide,. Lead Carbonate + Sulfuric Acid.
From www.kimyalab.net
Sülfürik Asit Nedir ? Sülfürik Asit Formülü ? Sülfürik Asit Lead Carbonate + Sulfuric Acid The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone. Lead Carbonate + Sulfuric Acid.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate + Sulfuric Acid Learn how acids react with carbonates and hydrogencarbonates to produce carbon dioxide, water and salts. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Lead sulfate is not soluble. Lead Carbonate + Sulfuric Acid.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate + Sulfuric Acid Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The chemical equation for the reaction between lead carbonate (pbco3) and sulfuric acid (h2so4) is: Enter an ionic equation and get its complete and net ionic. Lead Carbonate + Sulfuric Acid.
From www.researchgate.net
SEM images of lead carbonate hydroxide NSs prepared with 14 ratio of Lead Carbonate + Sulfuric Acid So a simple filtration will allow the separation lead. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Another method for preparing lead sulphate from lead carbonate is by reacting with sulphuric acid to directly produce lead sulphate,. See examples, equations and videos of the reactions. Enter. Lead Carbonate + Sulfuric Acid.
From www.slideshare.net
Reaction types Lead Carbonate + Sulfuric Acid Lead sulfate is not soluble in moderately concentrate or dilute sulfuric acid. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Another method for preparing lead sulphate from lead. Lead Carbonate + Sulfuric Acid.