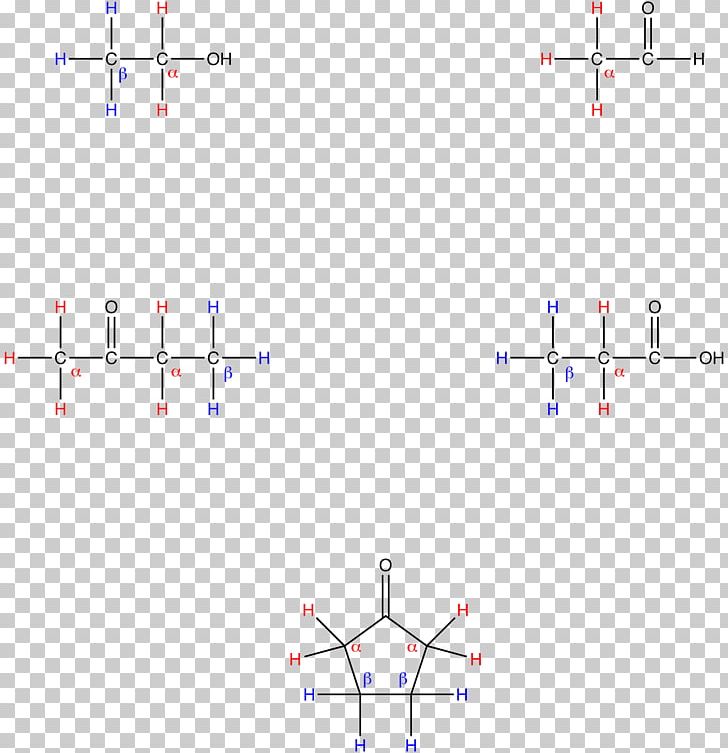
Alpha And Beta Face Chemistry . The carbon next to that is the beta The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β).
from imgbin.com
The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). The carbon next to that is the beta
Alpha And Beta Carbon Hydrogen Alpha Particle Chemistry PNG, Clipart
Alpha And Beta Face Chemistry The carbon next to that is the beta The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β).
From stock.adobe.com
Vector set of amber glass dropper bottles with AHA and BHA face serum Alpha And Beta Face Chemistry The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule. Alpha And Beta Face Chemistry.
From mytefaces.weebly.com
mytefaces Blog Alpha And Beta Face Chemistry The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The structure shown on the left side of. Alpha And Beta Face Chemistry.
From opentextbc.ca
10.6 Lattice Structures in Crystalline Solids Chemistry Alpha And Beta Face Chemistry The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the. Alpha And Beta Face Chemistry.
From www.youtube.com
Np Learning Review of Alpha and Beta Adrenergic Receptors 🧠 YouTube Alpha And Beta Face Chemistry In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The two isomeric forms are referred to by the greek letters alpha (α). Alpha And Beta Face Chemistry.
From byjus.com
What are alpha beta and gamma carbon atoms Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. In carbonyl compounds (ketones, aldehydes, esters and more) the. Alpha And Beta Face Chemistry.
From handwiki.org
ChemistryAlpha and beta carbon HandWiki Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α). Alpha And Beta Face Chemistry.
From www.pinterest.com
Properties of alpha, beta, and gamma particles Protons, Beta particle Alpha And Beta Face Chemistry The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The structures on the right side, with the oh group on the first carbon atom. Alpha And Beta Face Chemistry.
From hxeyqifop.blob.core.windows.net
Alpha Vs Beta Chemistry at Felicia Thompson blog Alpha And Beta Face Chemistry We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The structures on the right side,. Alpha And Beta Face Chemistry.
From www.youtube.com
Prochiral Centre Re and Si Faces Stereochemistry Organic Alpha And Beta Face Chemistry The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The two isomeric forms are referred to by the. Alpha And Beta Face Chemistry.
From imgbin.com
Alpha And Beta Carbon Hydrogen Alpha Particle Chemistry PNG, Clipart Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. In carbonyl compounds (ketones, aldehydes, esters. Alpha And Beta Face Chemistry.
From www.pinterest.co.uk
Alpha and beta glycosidic bonds Chemistry basics, Teaching chemistry Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other. Alpha And Beta Face Chemistry.
From stock.adobe.com
Structure of the haemoglobin ( hemoglobin ) molecule showing alpha and Alpha And Beta Face Chemistry The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The carbon next to that is the beta The structures on the right side, with the oh group on the first carbon atom pointed upward, is. Alpha And Beta Face Chemistry.
From www.cazypedia.org
Anomeric centre (alpha and beta) CAZypedia Alpha And Beta Face Chemistry We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected. Alpha And Beta Face Chemistry.
From chemistryscore.com
Beta (b) Position Learn Chemistry Online ChemistryScore Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule would. Alpha And Beta Face Chemistry.
From www.researchgate.net
1 Biophysical properties of beta, alpha, and Auger electron emitting Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The. Alpha And Beta Face Chemistry.
From www.youtube.com
Dehydration of Hydroxy Acids Heating of alpha(α), beta(β), gamma(γ Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other. Alpha And Beta Face Chemistry.
From www.slideserve.com
PPT Chapter 24 Nuclear Chemistry PowerPoint Presentation, free Alpha And Beta Face Chemistry We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The carbon next to that is the beta The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon. Alpha And Beta Face Chemistry.
From www.organicchemistrytutor.com
Nomenclature of Carbohydrates (the Fundamentals) — Organic Chemistry Tutor Alpha And Beta Face Chemistry The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon. Alpha And Beta Face Chemistry.
From www.masterorganicchemistry.com
9 Nomenclature Conventions To Know Master Organic Chemistry Alpha And Beta Face Chemistry The carbon next to that is the beta The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule would sit above the other as. Alpha And Beta Face Chemistry.
From www.masterorganicchemistry.com
Sugar and Carbohydrate Chemistry Definitions 29 Key Terms To Know Alpha And Beta Face Chemistry The carbon next to that is the beta The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of. Alpha And Beta Face Chemistry.
From www.masterorganicchemistry.com
Sugar and Carbohydrate Chemistry Definitions 29 Key Terms To Know Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). We have not learned about stereoisomerism quite yet, but you can still recognize that the. Alpha And Beta Face Chemistry.
From chemistnotes.com
Properties of alpha beta and gamma rays Chemistry Notes Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The carbon next to that is the beta The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). In carbonyl compounds (ketones,. Alpha And Beta Face Chemistry.
From psiberg.com
Alpha (α) Vs. Beta (β) decay A Key Comparison PSIBERG Alpha And Beta Face Chemistry The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face. Alpha And Beta Face Chemistry.
From sciencedrill.com
A Guide to Alpha Decay Including Equations and Uses Science Drill Alpha And Beta Face Chemistry In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. It is easy to. Alpha And Beta Face Chemistry.
From chemistryskills.com
Alpha (α), Beta (β), Gamma (γ) Carbon Atoms Chemistry Skills Alpha And Beta Face Chemistry The carbon next to that is the beta We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. In carbonyl compounds (ketones, aldehydes, esters and more). Alpha And Beta Face Chemistry.
From talsenchem.com
Unveiling Radiant Skin Harnessing the Power of Alpha and Beta Hydroxy Alpha And Beta Face Chemistry The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected downward, represent what is called the alpha (α) form. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). It is easy to imagine that one molecule would sit above the other as they. Alpha And Beta Face Chemistry.
From chem.libretexts.org
14.4 Reactions of Monosaccharides Chemistry LibreTexts Alpha And Beta Face Chemistry We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). The carbon next to. Alpha And Beta Face Chemistry.
From hxeyqifop.blob.core.windows.net
Alpha Vs Beta Chemistry at Felicia Thompson blog Alpha And Beta Face Chemistry In carbonyl compounds (ketones, aldehydes, esters and more) the alpha carbon is the carbon adjacent to the carbonyl. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The structure shown on the left side of figure 2, with the oh group on the first carbon atom projected. Alpha And Beta Face Chemistry.
From www.reddit.com
Anomeric Structure in Fischer, how to know alpha vs beta? r/Mcat Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The carbon next to that is the beta The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). The structure shown on. Alpha And Beta Face Chemistry.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Alpha And Beta Face Chemistry We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule would sit above the other as they approach,. Alpha And Beta Face Chemistry.
From www.slideshare.net
Alpha beta and gamma decay equations Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The two isomeric forms are referred to by the greek letters alpha (α) and beta (β). We have not learned about stereoisomerism quite yet, but you can still. Alpha And Beta Face Chemistry.
From stock.adobe.com
Alpha decay, beta decay and gamma decay equations. Nuclear chemistry Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. We have not learned about stereoisomerism quite yet, but you can still recognize that the bonding configuration on one carbon is different. In carbonyl compounds (ketones, aldehydes, esters. Alpha And Beta Face Chemistry.
From www.freepik.com
Premium Vector Alpha versus Beta Glucose chemistry vector Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. The two isomeric forms are referred. Alpha And Beta Face Chemistry.
From byjus.com
What is alpha, beta and Gama hydrogen atoms? Alpha And Beta Face Chemistry It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. In carbonyl compounds (ketones, aldehydes, esters. Alpha And Beta Face Chemistry.
From stock.adobe.com
alpha beta gamma symbols. Vector illustration isolated on white Alpha And Beta Face Chemistry The structures on the right side, with the oh group on the first carbon atom pointed upward, is the beta (β) form. It is easy to imagine that one molecule would sit above the other as they approach, and form bonds from one face of one molecule to one face of the other molecule. We have not learned about stereoisomerism. Alpha And Beta Face Chemistry.