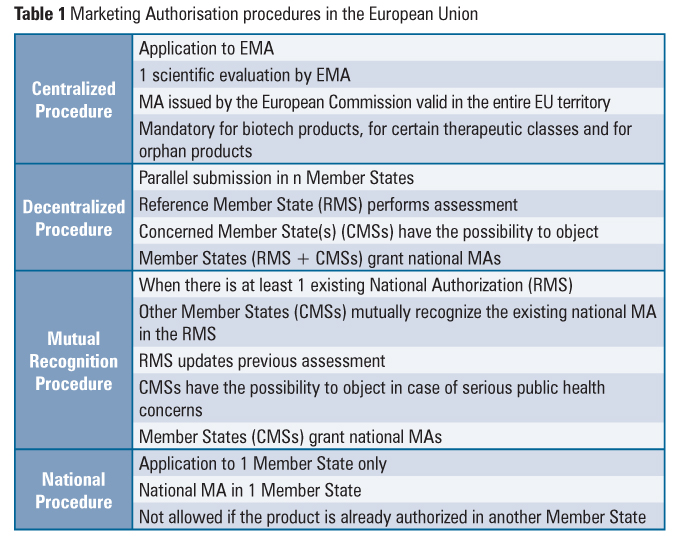
Blue Box Requirements Ema Human Medicines . the following are the specific requirements for the expression of the legal status in the boxed area: the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. annex i of this document lists, by member state, these national requirements. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. The information specific to a member state. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures.
from www.allfordrugs.com
the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. annex i of this document lists, by member state, these national requirements. the following are the specific requirements for the expression of the legal status in the boxed area: the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. The information specific to a member state.
Regulatory Approval Pathways All About Drugs
Blue Box Requirements Ema Human Medicines the following are the specific requirements for the expression of the legal status in the boxed area: The information specific to a member state. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. annex i of this document lists, by member state, these national requirements. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the following are the specific requirements for the expression of the legal status in the boxed area:
From pxhere.com
Free Images cute, love, small, couple, blue, together, ribbon, friend Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. The information specific to a member state. . Blue Box Requirements Ema Human Medicines.
From stickerbaker.com
I made an AI sticker of A blue box sitting to the left of a green chair Blue Box Requirements Ema Human Medicines annex i of this document lists, by member state, these national requirements. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. The information specific to a member state. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. details on. Blue Box Requirements Ema Human Medicines.
From www.bnn.in.th
Blue Box Case iPhone 14 Max (6.1) Clear Glitter with black camera frame Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. annex i of this document lists, by member state, these national requirements. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. the following are the specific requirements for the expression of the legal status in the boxed. Blue Box Requirements Ema Human Medicines.
From www.slideserve.com
PPT Collaboration between FDA and EMA PowerPoint Presentation, free Blue Box Requirements Ema Human Medicines annex i of this document lists, by member state, these national requirements. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the following are the specific requirements for the expression. Blue Box Requirements Ema Human Medicines.
From fastpng.com
Download A Blue Box With White Text And Blue Letters [100 Free] FastPNG Blue Box Requirements Ema Human Medicines annex i of this document lists, by member state, these national requirements. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. The information specific to a member state. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the following. Blue Box Requirements Ema Human Medicines.
From exyzolwfk.blob.core.windows.net
Product Label Requirements France at Simone Adkins blog Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. The information specific to a member state. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are. Blue Box Requirements Ema Human Medicines.
From www.youtube.com
Frank Zappa, Live Hammersmith Odeon 1978 (part 1) the Blue Box Blue Box Requirements Ema Human Medicines The information specific to a member state. the following are the specific requirements for the expression of the legal status in the boxed area: the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. annex i of this document lists, by member state, these national requirements. requirements on submissions for new marketing. Blue Box Requirements Ema Human Medicines.
From www.vecteezy.com
Medicine package box design. healthcare medicine box package creative Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. annex i of this document lists, by member state, these national requirements. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. requirements on submissions for new marketing authorisation applications within. Blue Box Requirements Ema Human Medicines.
From www.gopuff.com
L&M Blue Box Cigarettes 20ct Box 1pk goBooze Liquor Stores fast Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. annex i of this document lists, by member state, these national requirements. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given. Blue Box Requirements Ema Human Medicines.
From www.qinwandates.com
Diamond Blue Box Qinwan Dates Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. annex i of this document lists, by member state, these national requirements. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the european medicines agency (ema) makes guidance and templates. Blue Box Requirements Ema Human Medicines.
From pngtree.com
Blue Box That Looks Like A Gift Background, 3d Rendering Gift Box On Blue Box Requirements Ema Human Medicines The information specific to a member state. annex i of this document lists, by member state, these national requirements. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the following are the specific requirements for the expression of the legal status in the boxed area: the ‘blue box’ is mandatory on. Blue Box Requirements Ema Human Medicines.
From www.bluebox.co.jp
募集要項|採用情報NAVI|総合不動産会社ブルーボックス・ブルボ・BLUE BOX Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the following are the specific requirements for the expression of the legal status in the boxed area: details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline. Blue Box Requirements Ema Human Medicines.
From www.bybigplus.com
One Stop Packaging Material or Equipment Supplier in Selangor, Kuala Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. annex i of this document lists, by member state, these national requirements. the european. Blue Box Requirements Ema Human Medicines.
From picclick.co.uk
VINTAGE 70’S BLUE Box Preschool WindUp Toy Musical Television Red Blue Box Requirements Ema Human Medicines the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. The information specific to a member state. annex i of this document lists, by member state, these national requirements. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. requirements on. Blue Box Requirements Ema Human Medicines.
From www.falabella.com.pe
BALDE CANCHITA BLANCO BLUE BOX Blue Box Requirements Ema Human Medicines annex i of this document lists, by member state, these national requirements. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. The information specific. Blue Box Requirements Ema Human Medicines.
From www.lwd.org.kh
Pirata esegesi gruppo musicale blue box ema paio In qualsiasi momento Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. The. Blue Box Requirements Ema Human Medicines.
From exooyrgwm.blob.core.windows.net
First Aid Kits At Work Requirements at Donald Schrock blog Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. The information specific to a member state. . Blue Box Requirements Ema Human Medicines.
From www.allfordrugs.com
Regulatory Approval Pathways All About Drugs Blue Box Requirements Ema Human Medicines the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. The information specific to a member state. annex i of this document lists, by member state, these national requirements. details on the national requirements for the ‘blue box’. Blue Box Requirements Ema Human Medicines.
From lawprintpack.co.uk
Packaging Labelling Requirements in the European Union Blue Box Requirements Ema Human Medicines the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. annex i of this document lists, by member state, these national requirements. the following are the specific requirements for the expression. Blue Box Requirements Ema Human Medicines.
From www.smsupermalls.com
BLUE BOX SM City Tuguegarao SM Supermalls Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the following are the specific requirements for the expression of the legal status in the boxed area: details on the national. Blue Box Requirements Ema Human Medicines.
From thestreamable.com
Where to stream Blue Box (2021) online? Comparing 50+ Streaming Services Blue Box Requirements Ema Human Medicines details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. The information specific to a member state. requirements on submissions for new marketing authorisation applications. Blue Box Requirements Ema Human Medicines.
From victoriahouseco.com
THE BLUE BOX VALUES AT AMERICAN EXPRESS VIA Ken Chenault, 43 OFF Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the following are the specific requirements for the expression of the legal status in the boxed area: details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. The information specific to a. Blue Box Requirements Ema Human Medicines.
From www.dreamstime.com
European Medicines Agency Flag, EMA, European Union Stock Vector Blue Box Requirements Ema Human Medicines The information specific to a member state. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. the following are the specific requirements for the expression of the legal status in the boxed area: the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as. Blue Box Requirements Ema Human Medicines.
From pngtree.com
Blank Blue Box Isolated Box, Single, Present, Box PNG Transparent Image Blue Box Requirements Ema Human Medicines details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the following are the specific requirements for the expression of the legal status in the boxed area: the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as. Blue Box Requirements Ema Human Medicines.
From www.pinterest.com
Blue Box, Girl Photography, Manga, Anime, Anatomy, Photos, Quick, Hair Blue Box Requirements Ema Human Medicines the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. annex i of this document lists, by member state, these national requirements. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product. Blue Box Requirements Ema Human Medicines.
From pngtree.com
Indian Wedding Box With Line, Indian Wedding Box, Blue Wedding Blue Box Requirements Ema Human Medicines annex i of this document lists, by member state, these national requirements. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. the following are the specific requirements for the expression. Blue Box Requirements Ema Human Medicines.
From www.news-medical.net
Vaccine transport boxes for vaccine and pharmaceuticals solutions Get Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. the following are the specific requirements for the expression of the legal status in the boxed area: details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the european medicines agency. Blue Box Requirements Ema Human Medicines.
From dentalbluebox.com
Home Dental Blue Box Blue Box Requirements Ema Human Medicines requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. The information specific to a member state. the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the following are the specific requirements for the expression of the legal status in the. Blue Box Requirements Ema Human Medicines.
From www.shutterstock.com
Ema Approved Images Browse 91 Stock Photos & Vectors Free Download Blue Box Requirements Ema Human Medicines details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. annex i of this document lists, by member state, these national requirements. the following are the specific requirements for the expression. Blue Box Requirements Ema Human Medicines.
From exyzeavug.blob.core.windows.net
Medicine Labelling Information at Faith Chavez blog Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. The information specific to a member state. the following are the specific requirements for the. Blue Box Requirements Ema Human Medicines.
From dentalbluebox.com
Shop Dental Blue Box Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. annex i of this document lists, by member state, these national requirements. The information specific to a member state. the following are the specific requirements for the expression of the legal status in the boxed area:. Blue Box Requirements Ema Human Medicines.
From www.studio7thailand.com
Blue Box เคส iPhone 15 Pro Clear Shading edge with Black Blue Box Requirements Ema Human Medicines The information specific to a member state. the following are the specific requirements for the expression of the legal status in the boxed area: the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. annex i of this document lists, by member state, these national requirements.. Blue Box Requirements Ema Human Medicines.
From toolbox.eupati.eu
Information on medicinal products EUPATI Toolbox Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. the european medicines agency (ema) makes guidance and templates available to provide marketing authorisation. The information specific to a member state. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. . Blue Box Requirements Ema Human Medicines.
From www.theglobeandmail.com
NVDA Returning From The Elliott Wave Blue Box Area The Globe and Mail Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. annex i of this document lists, by member state, these national requirements. details on the national requirements for the ‘blue box’. Blue Box Requirements Ema Human Medicines.
From www.pharmaexcipients.com
Excipients labelling EMA information pharma excipients Blue Box Requirements Ema Human Medicines the ‘blue box’ is mandatory on the outer labelling of each centrally authorised human medicinal product as well as each parallel. requirements on submissions for new marketing authorisation applications within mrp, dcp and national procedures. details on the national requirements for the ‘blue box’ of centrally authorised medicinal products are given in the guideline on the. . Blue Box Requirements Ema Human Medicines.