Electroplating Copper To Silver . electroplating silver over copper takes a bit of skill and art to. You can weigh the coins before and after coating to find the mass of zinc Essentially, since silver is more reactive than many. at the copper electrode: electroplating an object with silver occurs based on certain chemical properties of some metals. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. silver plating, also known as sheffield plating, is a fusion process. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide.
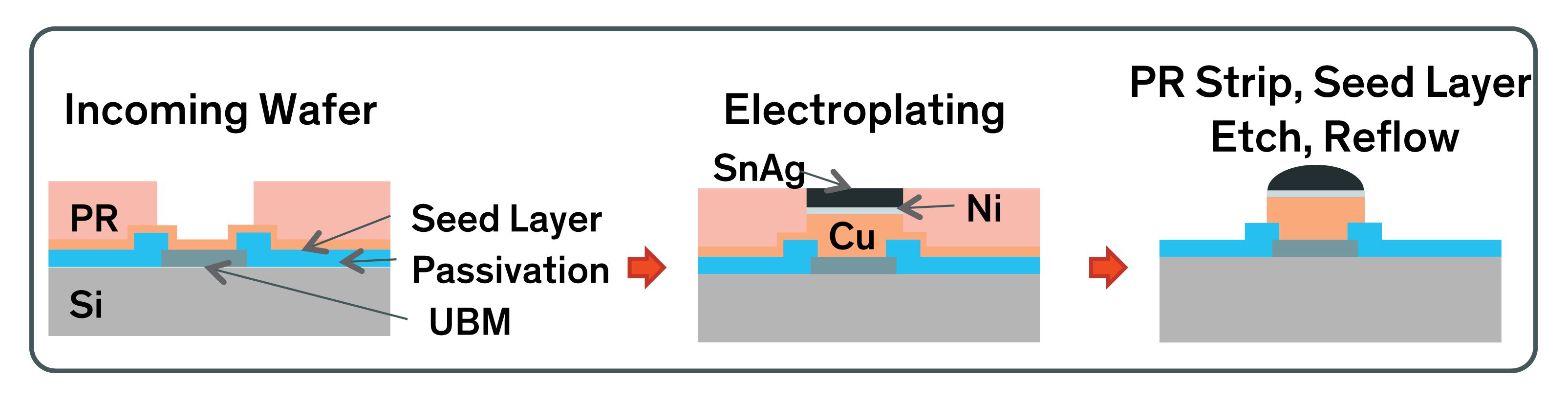
from www.dupont.com
To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating silver over copper takes a bit of skill and art to. silver plating, also known as sheffield plating, is a fusion process. Essentially, since silver is more reactive than many. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. electroplating an object with silver occurs based on certain chemical properties of some metals. You can weigh the coins before and after coating to find the mass of zinc at the copper electrode:
Copper pillar electroplating tutorial
Electroplating Copper To Silver To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. You can weigh the coins before and after coating to find the mass of zinc electroplating an object with silver occurs based on certain chemical properties of some metals. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating silver over copper takes a bit of skill and art to. at the copper electrode: silver plating, also known as sheffield plating, is a fusion process. Essentially, since silver is more reactive than many.
From ceg.edu.vn
Share more than 138 electroplating iron nail with copper ceg.edu.vn Electroplating Copper To Silver electroplating silver over copper takes a bit of skill and art to. silver plating, also known as sheffield plating, is a fusion process. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and silver plating of copper or copper alloys is a highly functional finish for. Electroplating Copper To Silver.
From sensorex.com
Electroplating The Process & Uses in Liquid Analysis Explained Sensorex Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. You can weigh the coins before and after coating to find the mass of zinc electroplating an object with silver occurs based on certain chemical properties of some metals. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high.. Electroplating Copper To Silver.
From www.youtube.com
BASICS OF ELECTROPLATING OF SILVER OVER COPPER AND REDOX REACTIONS Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. electroplating silver over copper takes a bit of skill and art to. Essentially, since silver is more reactive than many. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. at the copper electrode:. Electroplating Copper To Silver.
From www.pcbaaa.com
Electroplating copper A perfect choice for Ntype battery IBE Electroplating Copper To Silver copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. . Electroplating Copper To Silver.
From ytsab.mozello.com
ytsab What Is Electroplating Electroplating Copper To Silver electroplating silver over copper takes a bit of skill and art to. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating an object with silver. Electroplating Copper To Silver.
From www.wiringdraw.com
Draw A Simple Circuit Diagram To Show Electroplating And Answer The Electroplating Copper To Silver silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's. Electroplating Copper To Silver.
From www.researchgate.net
Basic setup of electroplating Copper. Download Scientific Diagram Electroplating Copper To Silver atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. electroplating an object with silver occurs based on certain chemical properties of some metals. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. Essentially, since silver is. Electroplating Copper To Silver.
From gioqrusrz.blob.core.windows.net
Copper Plate Electroplating Process at Maria Phelps blog Electroplating Copper To Silver copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. You can weigh the coins before and after coating to find the mass of zinc electroplating silver over copper takes a bit of skill and art to. at the copper electrode: silver plating of copper or copper alloys is. Electroplating Copper To Silver.
From www.chemedx.org
An Easy Copper Electroplating Demo for Your Redox Unit Chemical Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. You can weigh the coins before and after coating to find the mass of zinc copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. Essentially, since silver is more reactive than many. atoms of copper will transfer from. Electroplating Copper To Silver.
From www.youtube.com
Electroplating CopperPlate a Key YouTube Electroplating Copper To Silver copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. electroplating an object with silver occurs based on certain chemical properties of some metals. Essentially, since silver is more reactive than many. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the.. Electroplating Copper To Silver.
From narodnatribuna.info
Electroplating Diagram Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. at the copper electrode: in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating an object with silver occurs based on certain chemical properties of some metals. silver plating of copper or copper alloys is. Electroplating Copper To Silver.
From www.chemistrylearner.com
Electroplating Definition, Process, Example, and Equation Electroplating Copper To Silver You can weigh the coins before and after coating to find the mass of zinc Essentially, since silver is more reactive than many. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating silver over copper takes a bit of skill and art to. atoms of copper will. Electroplating Copper To Silver.
From brainly.in
Draw a welllabelled diagram of electroplating of copper on iron spoon Electroplating Copper To Silver at the copper electrode: in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. You can weigh the coins before and after coating to find the mass of. Electroplating Copper To Silver.
From byjus.com
9.What is electroplating with the help of diagram and word equation Electroplating Copper To Silver You can weigh the coins before and after coating to find the mass of zinc electroplating an object with silver occurs based on certain chemical properties of some metals. Essentially, since silver is more reactive than many. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide.. Electroplating Copper To Silver.
From www.dreamstime.com
Electroplating with Copper Using Copper Sulfate Electrolyte Stock Electroplating Copper To Silver at the copper electrode: copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and Essentially, since silver is more reactive than many. electroplating silver over copper takes a bit. Electroplating Copper To Silver.
From www.youtube.com
Easy StepbyStep Tutorial on Electroplating a CopperPlated Key YouTube Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. You can weigh the coins before and after coating to find the mass of zinc To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and atoms of copper will transfer from the copper metal and bond. Electroplating Copper To Silver.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Electroplating Copper To Silver Essentially, since silver is more reactive than many. electroplating an object with silver occurs based on certain chemical properties of some metals. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch. Electroplating Copper To Silver.
From all3dp.com
Electroplating 3D Prints All You Need to Know All3DP Pro Electroplating Copper To Silver You can weigh the coins before and after coating to find the mass of zinc in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and electroplating silver over copper. Electroplating Copper To Silver.
From www.youtube.com
How to Copper plate on to Aluminium YouTube Electroplating Copper To Silver To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. at the copper electrode: silver plating of copper or copper alloys is a highly functional finish for transferring heat. Electroplating Copper To Silver.
From www.youtube.com
What is Electroplating? Copper Electroplating YouTube Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and Essentially, since silver is more reactive than many. electroplating silver over copper takes a bit of skill and art to. electroplating an object with silver. Electroplating Copper To Silver.
From www.thoughtco.com
What Is Electroplating and How Does It Work? Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. You can weigh the coins before and after coating to find the mass of zinc atoms of copper will transfer from the copper metal and bond with the metal. Electroplating Copper To Silver.
From www.wiringdraw.com
Draw A Simple Circuit Diagram To Show Electroplating Electroplating Copper To Silver at the copper electrode: You can weigh the coins before and after coating to find the mass of zinc silver plating, also known as sheffield plating, is a fusion process. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. atoms of copper will transfer from the copper metal. Electroplating Copper To Silver.
From brainly.in
how will you electroplate a copper spoon with silver? drow the circuit Electroplating Copper To Silver You can weigh the coins before and after coating to find the mass of zinc Essentially, since silver is more reactive than many. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. electroplating an object with silver occurs based on certain chemical properties of some metals.. Electroplating Copper To Silver.
From classnotes.org.in
Electroplating Chemical effect of electric current, Class 8 Electroplating Copper To Silver at the copper electrode: silver plating, also known as sheffield plating, is a fusion process. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. electroplating silver over copper takes a bit of skill and art to. electroplating an object with silver occurs based on certain chemical. Electroplating Copper To Silver.
From suntechmachinery.en.made-in-china.com
Copper Wire Silver Electroplating Machine 0 to 300m/Min China Electroplating Copper To Silver You can weigh the coins before and after coating to find the mass of zinc silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. To avoid burn spots (spots. Electroplating Copper To Silver.
From www.youtube.com
Electroplating a key with copper The Real Chemist YouTube Electroplating Copper To Silver To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and electroplating an object with silver occurs based on certain chemical properties of some metals. at the copper electrode: Essentially, since silver is more reactive than many. You can weigh the coins before and after coating to find. Electroplating Copper To Silver.
From www.researchgate.net
Schematic representation of copper electroplating setup. Download Electroplating Copper To Silver silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. in silver electroplating of copper alloys, small differences in the alloy composition can have a. Electroplating Copper To Silver.
From philschatz.com
Electrolysis · Chemistry Electroplating Copper To Silver silver plating, also known as sheffield plating, is a fusion process. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. atoms of copper will transfer from. Electroplating Copper To Silver.
From ceg.edu.vn
Share more than 138 electroplating iron nail with copper ceg.edu.vn Electroplating Copper To Silver To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at least one inch apart and silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. electroplating silver over copper takes a bit of skill and art to. in silver electroplating. Electroplating Copper To Silver.
From brainly.in
you need a copper layer over an iron spoon.how to do it by using Electroplating Copper To Silver Essentially, since silver is more reactive than many. You can weigh the coins before and after coating to find the mass of zinc silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. silver plating, also known as sheffield plating, is a fusion process. in silver. Electroplating Copper To Silver.
From www.runsom.com
Here We Discuss Everything about Silver Plating Runsom Precision Electroplating Copper To Silver in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. To. Electroplating Copper To Silver.
From www.embibe.com
How can you electroplate an iron nail with copper Explain with the help Electroplating Copper To Silver in silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the. copper electroplating sees widespread usage in the manufacture of electrical and electronic devices, owing to copper's high. silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. . Electroplating Copper To Silver.
From brainly.in
Write an experiment to show the process of electroplating silver over Electroplating Copper To Silver silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. To avoid burn spots (spots where the copper accumulates too quickly), keep the two metals at. Electroplating Copper To Silver.
From www.dupont.com
Copper pillar electroplating tutorial Electroplating Copper To Silver electroplating silver over copper takes a bit of skill and art to. silver plating, also known as sheffield plating, is a fusion process. You can weigh the coins before and after coating to find the mass of zinc electroplating an object with silver occurs based on certain chemical properties of some metals. Essentially, since silver is more. Electroplating Copper To Silver.
From www.pcbaaa.com
Electroplating copper A perfect choice for Ntype battery IBE Electroplating Copper To Silver silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide. at the copper electrode: atoms of copper will transfer from the copper metal and bond with the metal you are plating to form a coat. electroplating an object with silver occurs based on certain chemical. Electroplating Copper To Silver.