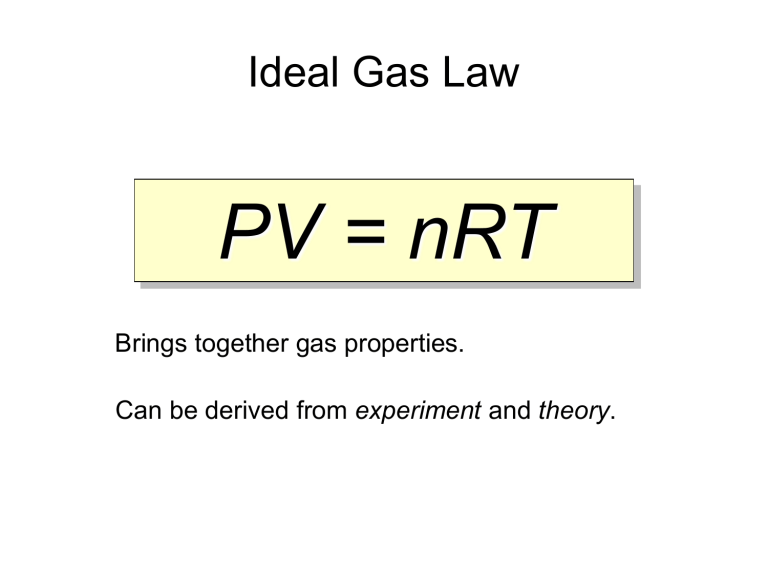
What Is A Gas Law Definition . It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The ideal gas law is also known as the general gas equation. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Boyle’s law —named for robert boyle —states.
from studylib.net
Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law —named for robert boyle —states. The ideal gas law is also known as the general gas equation. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature.
Ideal Gas Law
What Is A Gas Law Definition Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples What Is A Gas Law Definition The ideal gas law is also known as the general gas equation. Boyle’s law —named for robert boyle —states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas. What Is A Gas Law Definition.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2076011 What Is A Gas Law Definition Boyle’s law —named for robert boyle —states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal. What Is A Gas Law Definition.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID What Is A Gas Law Definition The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law is also known as the general gas equation. Boyle’s law describes the inverse proportional relationship between pressure and volume at a. What Is A Gas Law Definition.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID3630419 What Is A Gas Law Definition The ideal gas law is also known as the general gas equation. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between. What Is A Gas Law Definition.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is A Gas Law Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Boyle’s. What Is A Gas Law Definition.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free What Is A Gas Law Definition Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is also known as the. What Is A Gas Law Definition.
From www.youtube.com
Gas Laws Boyle's Law, Charles's Law, GayLussac’s Law and Avogadro's What Is A Gas Law Definition Boyle’s law —named for robert boyle —states. The ideal gas law is also known as the general gas equation. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. It is an equation of state of an ideal gas that relates pressure, volume, quantity. What Is A Gas Law Definition.
From lessonfullmattie.z21.web.core.windows.net
Gas Laws Involve What Three Variables What Is A Gas Law Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Gas laws, laws that relate the pressure, volume,. What Is A Gas Law Definition.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica What Is A Gas Law Definition Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.. What Is A Gas Law Definition.
From sciencenotes.org
Charles's Law Definition, Formula, Examples What Is A Gas Law Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The ideal gas law is also known as the general gas equation. The three fundamental gas laws discover the relationship of. What Is A Gas Law Definition.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas.. What Is A Gas Law Definition.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc What Is A Gas Law Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Boyle’s law —named for robert boyle —states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. It is an equation. What Is A Gas Law Definition.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What Is A Gas Law Definition Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is also known as the general gas equation. Boyle’s law —named for robert boyle —states. Ideal gas law, relation between the pressure. What Is A Gas Law Definition.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Is A Gas Law Definition Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named. What Is A Gas Law Definition.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What Is A Gas Law Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. The three fundamental gas laws discover the relationship. What Is A Gas Law Definition.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a. What Is A Gas Law Definition.
From gamesmartz.com
Ideal Gas Law Definition & Image GameSmartz What Is A Gas Law Definition Boyle’s law —named for robert boyle —states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Gas laws, laws that. What Is A Gas Law Definition.
From gamesmartz.com
Combined Gas Law Definition & Image GameSmartz What Is A Gas Law Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The ideal gas law is also known as the general gas equation. Ideal gas law, relation. What Is A Gas Law Definition.
From www.slideserve.com
PPT Introduction to the Gas Laws PowerPoint Presentation, free What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is also known as the general gas equation.. What Is A Gas Law Definition.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is also known as. What Is A Gas Law Definition.
From www.chemistrylearner.com
Gas Laws Definition, List, Equations, and Problems What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and. What Is A Gas Law Definition.
From www.britannica.com
Gas laws Definition & Facts Britannica What Is A Gas Law Definition The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is also known as the general gas equation. Gas laws, laws that relate the. What Is A Gas Law Definition.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Is A Gas Law Definition The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which. What Is A Gas Law Definition.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and. What Is A Gas Law Definition.
From www.chemistrylearner.com
Gas Laws Definition, List, Equations, and Problems What Is A Gas Law Definition Boyle’s law —named for robert boyle —states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas, a gas that conforms,. What Is A Gas Law Definition.
From www.worksheetsplanet.com
The Gas Laws What Is A Gas Law Definition Gas laws, laws that relate the pressure, volume, and temperature of a gas. The ideal gas law is also known as the general gas equation. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal. What Is A Gas Law Definition.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of. What Is A Gas Law Definition.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What Is A Gas Law Definition Boyle’s law —named for robert boyle —states. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Boyle’s law describes the inverse proportional relationship. What Is A Gas Law Definition.
From studylib.net
Ideal Gas Law What Is A Gas Law Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. Boyle’s. What Is A Gas Law Definition.
From sciencenotes.org
Boyle's Law Definition, Formula, Example What Is A Gas Law Definition The ideal gas law is also known as the general gas equation. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures. What Is A Gas Law Definition.
From www.sliderbase.com
Gas Laws Presentation Chemistry What Is A Gas Law Definition The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Boyle’s law —named for robert boyle —states. Boyle’s law describes the inverse proportional. What Is A Gas Law Definition.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What Is A Gas Law Definition Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for robert boyle —states. The ideal gas law is also known as the general gas equation. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas, a gas that conforms, in physical behavior, to a particular. What Is A Gas Law Definition.
From www.inspiritvr.com
GayLussac’s Law of Ideal Gasses Study Guide Inspirit What Is A Gas Law Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for robert boyle —states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low. What Is A Gas Law Definition.
From www.sliderbase.com
Gas Laws Presentation Chemistry What Is A Gas Law Definition The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a. What Is A Gas Law Definition.
From sciencenotes.org
Real Gas vs Ideal Gas What Is A Gas Law Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the. What Is A Gas Law Definition.