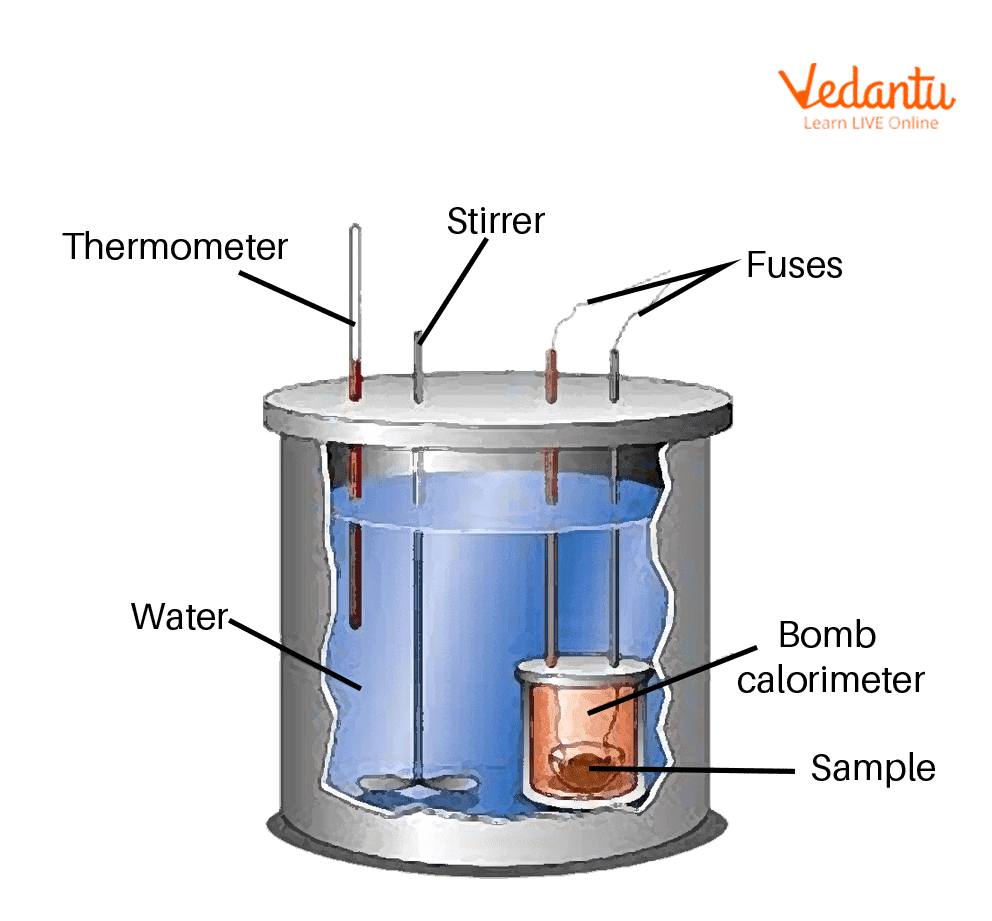
Conclusion Of Calorimetry Experiment . The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. have a chemistry practical on the calorimetry experiment? conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The calorimetry was used to determine the specific heat of aluminum metal. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed.
from www.vedantu.com
conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. have a chemistry practical on the calorimetry experiment? The calorimetry was used to determine the specific heat of aluminum metal. anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996).
Bomb Calorimeter Learn Important Terms and Concepts
Conclusion Of Calorimetry Experiment calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. have a chemistry practical on the calorimetry experiment? Ace your next chemistry assessment with this report on how to perform the calorimetry practical. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The calorimetry was used to determine the specific heat of aluminum metal. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings).
From saylordotorg.github.io
Calorimetry Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The calorimetry was used to determine the specific heat of aluminum metal. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. anything that is to be measured in. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The calorimetry was used to determine the specific heat of aluminum metal.. Conclusion Of Calorimetry Experiment.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. conclusion in conclusion, the results for the average heat capacity for all of these procedures. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. . Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? Ace your next chemistry assessment with this report on how to perform the calorimetry practical. anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). The heat of neutralization that is lost in the chemical reaction. Conclusion Of Calorimetry Experiment.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. The heat of neutralization that is lost in the chemical reaction (the system) is gained. Conclusion Of Calorimetry Experiment.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Conclusion Of Calorimetry Experiment calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. . Conclusion Of Calorimetry Experiment.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Conclusion Of Calorimetry Experiment conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. have a chemistry practical on the calorimetry experiment? The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). anything that is to be measured in. Conclusion Of Calorimetry Experiment.
From www.chegg.com
CALORIMETRY EXPERIMENT PRELAB... Conclusion Of Calorimetry Experiment The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). have a chemistry practical on the calorimetry experiment? The calorimetry was used to determine the specific heat of aluminum metal. in this experiment you will heat a known mass of a metal to a known. Conclusion Of Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Ace your next chemistry assessment with this report on how to perform the calorimetry practical. in this experiment you will heat a known mass of a metal. Conclusion Of Calorimetry Experiment.
From www.youtube.com
Calorimetry Experiment Demonstration YouTube Conclusion Of Calorimetry Experiment The calorimetry was used to determine the specific heat of aluminum metal. have a chemistry practical on the calorimetry experiment? conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The heat of neutralization. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. have a chemistry practical on the calorimetry experiment? Ace. Conclusion Of Calorimetry Experiment.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The calorimetry was used to determine the specific heat of aluminum metal. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings).. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. in this experiment you will heat a known mass of a metal to a known. Conclusion Of Calorimetry Experiment.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The calorimetry was used to determine the specific heat of aluminum metal. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. have a chemistry practical on the calorimetry experiment? calorimetry is based. Conclusion Of Calorimetry Experiment.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). The calorimetry was used to determine the specific heat of aluminum metal. calorimetry is based on the first law of thermodynamics that states that energy. Conclusion Of Calorimetry Experiment.
From wingle.jp
😂 Bomb calorimeter experiment conclusion. Experiment 1 A3 Bomb Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The calorimetry was used to determine the specific heat of aluminum metal. . Conclusion Of Calorimetry Experiment.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. The calorimetry was used to determine the specific heat of aluminum metal. The heat of neutralization. Conclusion Of Calorimetry Experiment.
From www.youtube.com
The Boys’ Gas Calorimeter Experiment HWUDC Vlab YouTube Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? The calorimetry was used to determine the specific heat of aluminum metal. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. in this experiment you will heat a known mass of a metal to a known temperature and then transfer. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). in this experiment you will heat a known mass of a metal to a known temperature and. Conclusion Of Calorimetry Experiment.
From www.studocu.com
P calorimetry 25 lab report StuDocu Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic.. Conclusion Of Calorimetry Experiment.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Conclusion Of Calorimetry Experiment conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. anything that is to be measured in terms of heat being generated and exchanged. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). Ace your next chemistry assessment with this report on how to perform the calorimetry practical. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic.. Conclusion Of Calorimetry Experiment.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. have a chemistry practical on the calorimetry experiment? The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The calorimetry was used to determine the specific heat of aluminum metal. . Conclusion Of Calorimetry Experiment.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. calorimetry is based on the first law of thermodynamics that states that energy cannot. Conclusion Of Calorimetry Experiment.
From exooqotfj.blob.core.windows.net
Calorimeter Chemistry at Roberta Burgett blog Conclusion Of Calorimetry Experiment The calorimetry was used to determine the specific heat of aluminum metal. anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Conclusion Of Calorimetry Experiment.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Conclusion Of Calorimetry Experiment The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. conclusion in. Conclusion Of Calorimetry Experiment.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Conclusion Of Calorimetry Experiment The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). anything that is to be measured in terms of heat being generated and exchanged in an environment can be used in calorimetry (chieh, 1996). in this experiment you will heat a known mass of a. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The calorimetry was used to determine the specific heat of aluminum metal. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. The heat of neutralization that is lost in the chemical reaction (the system) is. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
calorimetry experiment lab report conclusion Conclusion Of Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The calorimetry was used to determine the specific heat of aluminum metal. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Ace your next chemistry. Conclusion Of Calorimetry Experiment.
From www.pdfprof.com
experiment 25 calorimetry lab report Conclusion Of Calorimetry Experiment have a chemistry practical on the calorimetry experiment? in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. The calorimetry was used to determine. Conclusion Of Calorimetry Experiment.
From www.studocu.com
CoffeeCup Calorimetry Experiment Lab Report with answers and solutions Conclusion Of Calorimetry Experiment conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. have a chemistry practical on the calorimetry experiment? The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Ace your next chemistry assessment with this report. Conclusion Of Calorimetry Experiment.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Conclusion Of Calorimetry Experiment The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). have a chemistry practical on the calorimetry experiment? in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. anything that. Conclusion Of Calorimetry Experiment.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Conclusion Of Calorimetry Experiment calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Ace your next chemistry assessment with this report on how to perform the calorimetry practical. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). have a. Conclusion Of Calorimetry Experiment.