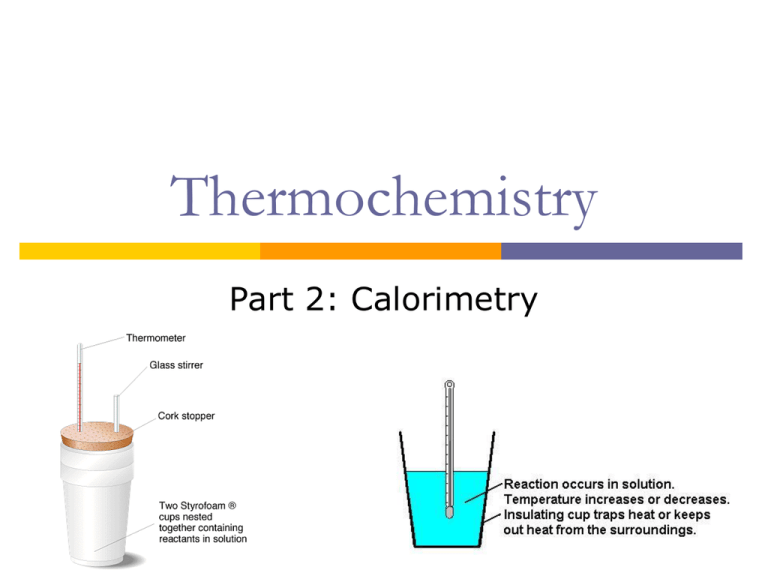
Calorimetry Definition Igcse . The apparatus used to measure the change in energy is called a calorimeter. By knowing the change in. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. A method which allows enthalpies of a reaction to be determined by experiment. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. What equation would you use to.
from studylib.net
3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. The apparatus used to measure the change in energy is called a calorimeter. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. A known amount of water is placed in a glass or copper container. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment.
Calorimetry
Calorimetry Definition Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. A method which allows enthalpies of a reaction to be determined by experiment. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. By knowing the change in. A known amount of water is placed in a glass or copper container. The apparatus used to measure the change in energy is called a calorimeter. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. What equation would you use to.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Definition Igcse Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The apparatus used to measure the change in energy is called a calorimeter. A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Revision notes. Calorimetry Definition Igcse.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimetry Definition Igcse A known amount of water is placed in a glass or copper container. A method which allows enthalpies of a reaction to be determined by experiment. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts. Calorimetry Definition Igcse.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Definition Igcse The apparatus used to measure the change in energy is called a calorimeter. By knowing the change in. What equation would you use to. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. 3.2 describe simple. Calorimetry Definition Igcse.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Definition Igcse A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. What equation would you use to. A method which allows enthalpies of a reaction to. Calorimetry Definition Igcse.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Definition Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3:02 describe. Calorimetry Definition Igcse.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Definition Igcse What equation would you use to. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. A method which allows enthalpies of a reaction to be determined by experiment. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams.. Calorimetry Definition Igcse.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Definition Igcse The apparatus used to measure the change in energy is called a calorimeter. What equation would you use to. A known amount of water is placed in a glass or copper container. By knowing the change in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Revision notes on calorimetry for. Calorimetry Definition Igcse.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Definition Igcse A known amount of water is placed in a glass or copper container. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by. Calorimetry Definition Igcse.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Definition Igcse What equation would you use to. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry. Calorimetry Definition Igcse.
From studylib.net
Calorimetry Calorimetry Definition Igcse By knowing the change in. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. A method which allows enthalpies of a reaction to be determined by experiment. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. What equation would you use. Calorimetry Definition Igcse.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimetry Definition Igcse What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. A known amount of water is placed in a glass or copper container. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written. Calorimetry Definition Igcse.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Definition Igcse 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. The apparatus used to measure the change in energy is called a calorimeter. What equation would you use to. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Calorimetry is the process of measuring the amount of. Calorimetry Definition Igcse.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimetry Definition Igcse A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A method which allows enthalpies of a reaction to be determined by experiment. The apparatus used to measure the change in energy is called a calorimeter. 3:02 describe simple calorimetry experiments for. Calorimetry Definition Igcse.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Definition Igcse Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. A method which allows enthalpies of a reaction to be determined by experiment. By knowing the change in. 4.11 describe simple calorimetry experiments. Calorimetry Definition Igcse.
From studylib.net
Calorimetry Calorimetry Definition Igcse What equation would you use to. The apparatus used to measure the change in energy is called a calorimeter. A known amount of water is placed in a glass or copper container. A method which allows enthalpies of a reaction to be determined by experiment. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts. Calorimetry Definition Igcse.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Definition Igcse By knowing the change in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. The apparatus used to measure the change in energy is called a calorimeter. A known amount of water. Calorimetry Definition Igcse.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Definition Igcse Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. 4.11. Calorimetry Definition Igcse.
From www.youtube.com
CALORIMETRY Calorimetry Class 10 ICSE Calorimetry Theory and Calorimetry Definition Igcse By knowing the change in. A known amount of water is placed in a glass or copper container. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 3.2 describe simple calorimetry experiments for reactions such as combustion,. Calorimetry Definition Igcse.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Definition Igcse By knowing the change in. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. A known amount of water is placed in a glass or copper container. What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment. Calorimetry is the process of measuring. Calorimetry Definition Igcse.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Definition Igcse By knowing the change in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Revision notes on calorimetry for. Calorimetry Definition Igcse.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic18_energetics_004_calorimetrylabelled Calorimetry Definition Igcse The apparatus used to measure the change in energy is called a calorimeter. A method which allows enthalpies of a reaction to be determined by experiment. By knowing the change in. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 4.11 describe simple calorimetry experiments for reactions such as combustion,. Calorimetry Definition Igcse.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimetry Definition Igcse Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts. Calorimetry Definition Igcse.
From www.youtube.com
7. Edexcel GCSE Biology Calorimetry YouTube Calorimetry Definition Igcse Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. The apparatus used to measure the change in energy is called a calorimeter.. Calorimetry Definition Igcse.
From www.studypool.com
SOLUTION Calorimetry Biology Revision with Questions (GCSE Edexcel Calorimetry Definition Igcse By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. What equation would you use to. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry Definition Igcse.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Definition Igcse By knowing the change in. A method which allows enthalpies of a reaction to be determined by experiment. What equation would you use to. A known amount of water is placed in a glass or copper container. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. 3.2 describe simple calorimetry experiments for. Calorimetry Definition Igcse.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Definition Igcse 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. The apparatus used to measure the change in energy is called a calorimeter. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and. Calorimetry Definition Igcse.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Definition Igcse A known amount of water is placed in a glass or copper container. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A method which allows enthalpies of a. Calorimetry Definition Igcse.
From www.indirectcalorimetry.net
Understanding Indirect Calorimetry Indirect Calorimetry Calorimetry Definition Igcse What equation would you use to. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The apparatus used to measure the change in energy is called a calorimeter. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3.2 describe simple calorimetry experiments. Calorimetry Definition Igcse.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Definition Igcse The apparatus used to measure the change in energy is called a calorimeter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. A method which allows enthalpies of a reaction to be determined. Calorimetry Definition Igcse.
From www.youtube.com
Numericals of Calorimetry Calorimetry Class 10 Physics ICSE YouTube Calorimetry Definition Igcse Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. By knowing the change in. What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment.. Calorimetry Definition Igcse.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Calorimetry Definition Igcse 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A known amount of water is placed in a glass or copper container. A method which allows enthalpies of a reaction to be determined by experiment. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. By knowing. Calorimetry Definition Igcse.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Definition Igcse Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. A method which allows enthalpies of a reaction to be determined by experiment. A known amount of water is placed in a glass or copper container. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving. Calorimetry Definition Igcse.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Definition Igcse What equation would you use to. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A method which allows enthalpies of a reaction to be determined by experiment. A known amount of water is placed in a glass or copper container. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the. Calorimetry Definition Igcse.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Definition Igcse The apparatus used to measure the change in energy is called a calorimeter. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. A known amount of water is placed in a glass or copper container. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Revision. Calorimetry Definition Igcse.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Definition Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation calorimetry allows for the. Revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. A method which allows enthalpies of. Calorimetry Definition Igcse.