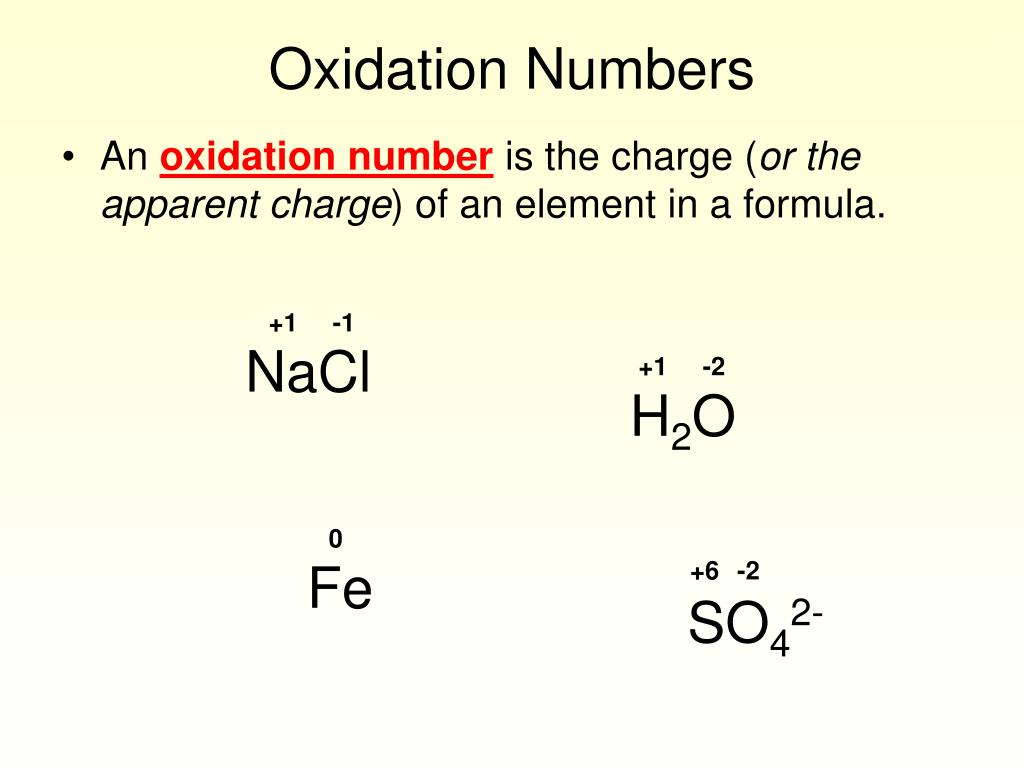
Chloride Ion Oxidation Number . Each atom that participates in an oxidation. The c atom has no valence electrons. Enter the formula of a chemical compound to find the oxidation number of each element. The sum of the oxidation numbers is zero. For an ion, the oxidation state is the charge. In \(\ce{ca_3p_2}\), the calcium is. A net ionic charge can be specified at the end of the. It has lost four electrons, so its oxidation number is +4. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The oxidation number of sodium in the na + ion is +1, for example, and. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. Don't forget that there are 2. The oxidation number of simple ions is equal to the charge on the ion.
from www.slideserve.com
Each atom that participates in an oxidation. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. A net ionic charge can be specified at the end of the. For an ion, the oxidation state is the charge. In \(\ce{ca_3p_2}\), the calcium is. It has lost four electrons, so its oxidation number is +4. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. The c atom has no valence electrons. Don't forget that there are 2. The sum of the oxidation numbers is zero.
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015
Chloride Ion Oxidation Number The c atom has no valence electrons. The oxidation number of sodium in the na + ion is +1, for example, and. It has lost four electrons, so its oxidation number is +4. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. In \(\ce{ca_3p_2}\), the calcium is. The oxidation number of simple ions is equal to the charge on the ion. The c atom has no valence electrons. A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. For an ion, the oxidation state is the charge. The sum of the oxidation numbers is zero. Each atom that participates in an oxidation. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. Don't forget that there are 2.
From www.youtube.com
How to find the Oxidation Number for Cl in the ClO ion. (Hypochlorite ion) YouTube Chloride Ion Oxidation Number Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. For an ion, the oxidation state is the charge. Don't forget that there are 2. The sum of the oxidation numbers is zero. A net ionic charge can be specified at the end of the. Each. Chloride Ion Oxidation Number.
From www.toppr.com
Oxidation number of Cl in KClO3 Chloride Ion Oxidation Number The oxidation number of sodium in the na + ion is +1, for example, and. Don't forget that there are 2. It has lost four electrons, so its oxidation number is +4. The c atom has no valence electrons. The oxidation number of simple ions is equal to the charge on the ion. The sum of the oxidation numbers is. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Chloride Ion Oxidation Number For an ion, the oxidation state is the charge. Don't forget that there are 2. It has lost four electrons, so its oxidation number is +4. The oxidation number of simple ions is equal to the charge on the ion. Enter the formula of a chemical compound to find the oxidation number of each element. The c atom has no. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Chloride Ion Oxidation Number The oxidation number of sodium in the na + ion is +1, for example, and. In \(\ce{ca_3p_2}\), the calcium is. The oxidation number of simple ions is equal to the charge on the ion. A net ionic charge can be specified at the end of the. For an ion, the oxidation state is the charge. The sum of the oxidation. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT 1. Give the oxidation number for the indicated element S in H 2 S 2 O 7 PowerPoint Chloride Ion Oxidation Number Each atom that participates in an oxidation. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. The sum of the oxidation numbers is zero. It has lost four electrons, so its oxidation number is +4. The c atom has no valence electrons. For an ion,. Chloride Ion Oxidation Number.
From www.bartleby.com
Answered 123. The oxidation numbers of P, S, and… bartleby Chloride Ion Oxidation Number In \(\ce{ca_3p_2}\), the calcium is. For an ion, the oxidation state is the charge. The oxidation number of sodium in the na + ion is +1, for example, and. Enter the formula of a chemical compound to find the oxidation number of each element. The element that is oxidized increases its oxidation number, and the element that is reduced decreases. Chloride Ion Oxidation Number.
From www.numerade.com
SOLVED Give the oxidation numbers for the underlined atoms in the following molecules and ions Chloride Ion Oxidation Number The oxidation number of sodium in the na + ion is +1, for example, and. The oxidation number of simple ions is equal to the charge on the ion. Don't forget that there are 2. The c atom has no valence electrons. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation. Chloride Ion Oxidation Number.
From www.youtube.com
How To Calculate Oxidation Numbers Basic Introduction YouTube Chloride Ion Oxidation Number A net ionic charge can be specified at the end of the. It has lost four electrons, so its oxidation number is +4. Each atom that participates in an oxidation. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. In \(\ce{ca_3p_2}\), the calcium is. The. Chloride Ion Oxidation Number.
From www.onlinechemistrytutor.net
chlorine001 Online Chemistry Tutor Chloride Ion Oxidation Number A net ionic charge can be specified at the end of the. Each atom that participates in an oxidation. The oxidation number of simple ions is equal to the charge on the ion. Enter the formula of a chemical compound to find the oxidation number of each element. The c atom has no valence electrons. Oxidation number, the total number. Chloride Ion Oxidation Number.
From elchoroukhost.net
Periodic Table With Oxidation Numbers And Names Elcho Table Chloride Ion Oxidation Number For an ion, the oxidation state is the charge. It has lost four electrons, so its oxidation number is +4. The c atom has no valence electrons. The oxidation number of sodium in the na + ion is +1, for example, and. Oxidation number, the total number of electrons that an atom either gains or loses in order to form. Chloride Ion Oxidation Number.
From byjus.com
oxidation number of Both Cl in Ca(OCl)Cl Chloride Ion Oxidation Number The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The c atom has no valence electrons. A net ionic charge can be specified at the end of the. Each atom that participates in an oxidation. The sum of the oxidation numbers is zero. Enter the formula of a chemical compound. Chloride Ion Oxidation Number.
From www.youtube.com
Oxidation numbers of chlorine in various compounds and ions YouTube Chloride Ion Oxidation Number A net ionic charge can be specified at the end of the. The oxidation number of sodium in the na + ion is +1, for example, and. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The sum of the oxidation numbers is zero. It has lost four electrons, so. Chloride Ion Oxidation Number.
From linatusims.blogspot.com
Oxidation Number of Chlorine LinatuSims Chloride Ion Oxidation Number In \(\ce{ca_3p_2}\), the calcium is. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. Don't forget that there are 2. For an ion, the oxidation state is the charge. It has lost four electrons, so its oxidation number is +4. Oxidation number,. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers & Redox Reactions PowerPoint Presentation ID5676215 Chloride Ion Oxidation Number The oxidation number of simple ions is equal to the charge on the ion. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. It has lost four electrons, so its oxidation number is +4. Enter the formula of a chemical compound to find the oxidation number of each element. The. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID1951364 Chloride Ion Oxidation Number The oxidation number of sodium in the na + ion is +1, for example, and. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The sum of the oxidation numbers is zero. It has lost four electrons, so its oxidation number is +4. Don't forget that there are 2. For. Chloride Ion Oxidation Number.
From brainly.com
What is the oxidation number for Cl in the molecule KCLO2 Chloride Ion Oxidation Number The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. A net ionic charge can be specified at the end of the. Don't forget that there are. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Unit 5 Part 2 Redox Reactions and Electrochemistry PowerPoint Presentation ID4566213 Chloride Ion Oxidation Number The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of sodium in the na + ion is +1, for example, and. Enter the formula of a chemical compound to find the oxidation number of each element. The element that is oxidized increases its oxidation number, and the element that is reduced decreases. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT CHEMISTRY OF COORDINATION COMPOUNDS PowerPoint Presentation, free download ID9496505 Chloride Ion Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. Don't forget that there are 2. Each atom that participates in an oxidation. In \(\ce{ca_3p_2}\), the calcium is. The sum of the oxidation numbers is zero. The c atom has no valence electrons. It has lost four electrons, so its oxidation number is +4. The. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Chapter 72 Oxidation Numbers PowerPoint Presentation, free download ID5363940 Chloride Ion Oxidation Number Don't forget that there are 2. A net ionic charge can be specified at the end of the. For an ion, the oxidation state is the charge. It has lost four electrons, so its oxidation number is +4. The sum of the oxidation numbers is zero. Each atom that participates in an oxidation. The c atom has no valence electrons.. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Assigning Oxidation Numbers in Covalent Compounds PowerPoint Presentation ID2854779 Chloride Ion Oxidation Number It has lost four electrons, so its oxidation number is +4. Each atom that participates in an oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. For an ion, the oxidation state is the charge. The oxidation number of sodium in the na + ion is +1, for example, and. The oxidation number. Chloride Ion Oxidation Number.
From sciencenotes.org
How to Assign Oxidation Numbers Chloride Ion Oxidation Number In \(\ce{ca_3p_2}\), the calcium is. Don't forget that there are 2. The sum of the oxidation numbers is zero. The oxidation number of sodium in the na + ion is +1, for example, and. The oxidation number of simple ions is equal to the charge on the ion. It has lost four electrons, so its oxidation number is +4. Each. Chloride Ion Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chloride Ion Oxidation Number Each atom that participates in an oxidation. For an ion, the oxidation state is the charge. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of sodium in the na + ion is +1, for example, and. It has lost four electrons, so its oxidation number is +4. A net ionic charge. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Chloride Ion Oxidation Number The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The oxidation number of sodium in the na + ion is +1, for example, and. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. The c. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2395087 Chloride Ion Oxidation Number The c atom has no valence electrons. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. For an ion, the oxidation state is the charge. It has lost four electrons, so its oxidation number is +4. Enter the formula of a chemical compound to find the oxidation number of each. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Chloride Ion Oxidation Number Each atom that participates in an oxidation. For an ion, the oxidation state is the charge. The oxidation number of simple ions is equal to the charge on the ion. The sum of the oxidation numbers is zero. The c atom has no valence electrons. Enter the formula of a chemical compound to find the oxidation number of each element.. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers & Redox Reactions PowerPoint Presentation ID5676215 Chloride Ion Oxidation Number Each atom that participates in an oxidation. Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. It has lost four electrons, so its oxidation number is +4. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Elements and Periodic Table PowerPoint Presentation, free download ID410846 Chloride Ion Oxidation Number It has lost four electrons, so its oxidation number is +4. Enter the formula of a chemical compound to find the oxidation number of each element. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. Each atom that participates in an oxidation. In \(\ce{ca_3p_2}\), the calcium is. The oxidation number. Chloride Ion Oxidation Number.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Chloride Ion Oxidation Number It has lost four electrons, so its oxidation number is +4. The oxidation number of sodium in the na + ion is +1, for example, and. A net ionic charge can be specified at the end of the. Don't forget that there are 2. Each atom that participates in an oxidation. The sum of the oxidation numbers is zero. In. Chloride Ion Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for N in NH4Cl (Ammonium chloride) YouTube Chloride Ion Oxidation Number Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. A net ionic charge can be specified at the end of the. Don't forget that there are 2. For an ion, the oxidation state is the charge. It has lost four electrons, so its oxidation number. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Chloride Ion Oxidation Number Don't forget that there are 2. The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. The sum of the oxidation numbers is zero. The oxidation number of sodium in the na + ion is +1, for example, and. It has lost four electrons, so its oxidation number is +4. Each. Chloride Ion Oxidation Number.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 7 Oxidation Numbers PowerPoint Presentation ID2300153 Chloride Ion Oxidation Number The element that is oxidized increases its oxidation number, and the element that is reduced decreases its oxidation number. It has lost four electrons, so its oxidation number is +4. Each atom that participates in an oxidation. The sum of the oxidation numbers is zero. The c atom has no valence electrons. Don't forget that there are 2. A net. Chloride Ion Oxidation Number.
From www.youtube.com
How to calculate the oxidation number of Cl in HClO2 (chlorous acid). YouTube Chloride Ion Oxidation Number Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. For an ion, the oxidation state is the charge. A net ionic charge can be specified at the end of the. In \(\ce{ca_3p_2}\), the calcium is. The oxidation number of sodium in the na + ion. Chloride Ion Oxidation Number.
From www.sliderbase.com
Oxidation Numbers and names Chloride Ion Oxidation Number In \(\ce{ca_3p_2}\), the calcium is. Don't forget that there are 2. The oxidation number of simple ions is equal to the charge on the ion. It has lost four electrons, so its oxidation number is +4. The c atom has no valence electrons. A net ionic charge can be specified at the end of the. The element that is oxidized. Chloride Ion Oxidation Number.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Chloride Ion Oxidation Number Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. Don't forget that there are 2. The oxidation number of simple ions is equal to the charge on the ion. For an ion, the oxidation state is the charge. Enter the formula of a chemical compound. Chloride Ion Oxidation Number.
From www.youtube.com
How to Find Oxidation Numbers (Rules and Examples) YouTube Chloride Ion Oxidation Number It has lost four electrons, so its oxidation number is +4. Don't forget that there are 2. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of simple ions is equal to the charge on the ion. In \(\ce{ca_3p_2}\), the calcium is. The c atom has no valence electrons. The oxidation. Chloride Ion Oxidation Number.