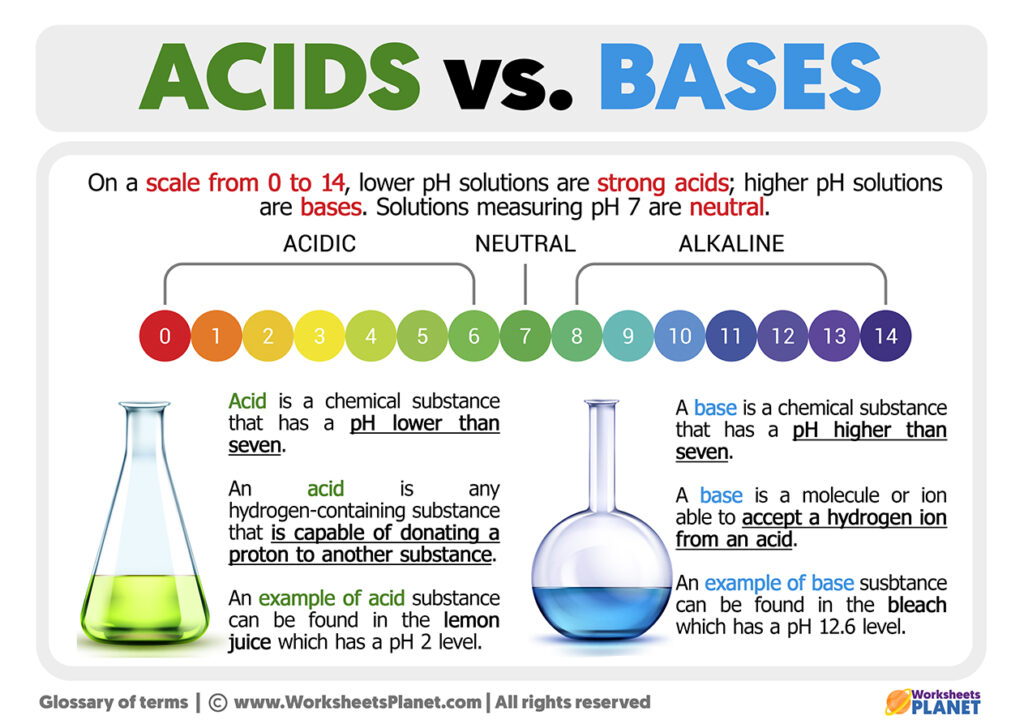
What Are Some Properties Of An Acid . Other acids are weak electrolytes that exist. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Acids have a sour taste. A base is a substance that can. A dilute acid has a sour or sharp taste. When reacted with metals, these substances produce hydrogen gas. Lemons, vinegar, and sour candies all contain acids. Vinegar, lemon juice, car batteries. Acids are sour in taste. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Their ph values are always less than 7. Acids are corrosive in nature. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions.
from www.worksheetsplanet.com
some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. — properties of acids. When reacted with metals, these substances produce hydrogen gas. Lemons, vinegar, and sour candies all contain acids. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Other acids are weak electrolytes that exist. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Acids are sour in taste.
Acid and Bases Differences
What Are Some Properties Of An Acid — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. — properties of acids. Other acids are weak electrolytes that exist. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Acids are corrosive in nature. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Vinegar, lemon juice, car batteries. Their ph values are always less than 7. When reacted with metals, these substances produce hydrogen gas. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Acids can be harmful and corrosive. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. Acids are sour in taste. A base is a substance that can.
From www.slideshare.net
Acids and bases ppt notes What Are Some Properties Of An Acid — properties of acids. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Acids are sour in taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus.. What Are Some Properties Of An Acid.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. When reacted with metals, these substances produce hydrogen gas. Lemons, vinegar, and sour candies all contain acids. Acids are corrosive in nature.. What Are Some Properties Of An Acid.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Are Some Properties Of An Acid When reacted with metals, these substances produce hydrogen gas. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Acids are sour in taste. They are good conductors of electricity. Acids are corrosive in nature. Vinegar, lemon juice, car batteries. — properties of acids. Aqueous solutions of acids are electrolytes, meaning that. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Are Some Properties Of An Acid Acids have a sour taste. Acids are sour in taste. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. A dilute acid has a sour or sharp taste. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. A base. What Are Some Properties Of An Acid.
From sharedocnow.blogspot.com
Physical Properties Of An Acid sharedoc What Are Some Properties Of An Acid Acids are sour in taste. Acids can be harmful and corrosive. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Acids are corrosive in nature. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A. What Are Some Properties Of An Acid.
From www.teachoo.com
Acids and it's Properties Definition [with Flowchart and Examples] What Are Some Properties Of An Acid you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Vinegar, lemon juice, car batteries. They are good conductors of electricity. Aqueous solutions of acids are electrolytes, meaning. What Are Some Properties Of An Acid.
From www.thoughtco.com
10 Common Acids and Chemical Structures What Are Some Properties Of An Acid — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Vinegar, lemon juice, car batteries. Lemons, vinegar, and sour candies all contain acids. Acids have a sour taste. Acids are sour in taste. Acids are corrosive in nature. Other acids are weak electrolytes that exist. Their ph values are. What Are Some Properties Of An Acid.
From dxonjrmrf.blob.core.windows.net
What Are The General Properties Of Acid at Leon Ussery blog What Are Some Properties Of An Acid Lemons, vinegar, and sour candies all contain acids. Acids are corrosive in nature. A dilute acid has a sour or sharp taste. They are good conductors of electricity. When reacted with metals, these substances produce hydrogen gas. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. Vinegar, lemon juice, car batteries. — properties of. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID9013474 What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Acids are sour in taste. Lemons, vinegar, and sour candies all contain acids. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. you will learn what is an acid, the. What Are Some Properties Of An Acid.
From sciencestruck.com
Properties of Acids Science Struck What Are Some Properties Of An Acid — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Acids have a sour taste. They are good conductors of. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Are Some Properties Of An Acid A base is a substance that can. Vinegar, lemon juice, car batteries. Acids have a sour taste. Lemons, vinegar, and sour candies all contain acids. Acids are sour in taste. Their ph values are always less than 7. Other acids are weak electrolytes that exist. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. — acid,. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids and Bases Introduction PowerPoint Presentation, free What Are Some Properties Of An Acid you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Acids have a sour taste. — properties of acids. They are good conductors of electricity. Acids are corrosive in nature. Other acids are weak electrolytes that exist. When reacted with metals, these substances produce. What Are Some Properties Of An Acid.
From sciencenotes.org
Household Acids and Bases What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A base is a substance that can. Other acids are weak electrolytes that exist. Lemons, vinegar, and sour candies all contain acids. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus.. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids and Alkalis PowerPoint Presentation, free download ID3411390 What Are Some Properties Of An Acid Their ph values are always less than 7. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A base is a substance that can. They are good conductors of electricity. Lemons, vinegar, and sour candies all contain acids. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. When reacted. What Are Some Properties Of An Acid.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary What Are Some Properties Of An Acid Lemons, vinegar, and sour candies all contain acids. Acids can be harmful and corrosive. Acids have a sour taste. Other acids are weak electrolytes that exist. A base is a substance that can. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. — acid, any substance that in water solution tastes sour, changes the colour of. What Are Some Properties Of An Acid.
From www.worksheetsplanet.com
Acid and Bases Differences What Are Some Properties Of An Acid They are good conductors of electricity. Lemons, vinegar, and sour candies all contain acids. Acids are corrosive in nature. Other acids are weak electrolytes that exist. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — acid, any substance. What Are Some Properties Of An Acid.
From www.worksheetsplanet.com
Properties of Acids Characteristics of Acids What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A base is a substance that can. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. Lemons, vinegar, and sour candies all contain acids. A dilute acid has a sour or sharp taste. Acids can be harmful and. What Are Some Properties Of An Acid.
From www.onlinebiologynotes.com
Properties of amino acids physical and chemical Online Biology Notes What Are Some Properties Of An Acid — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. — properties of acids. A dilute acid has a sour or sharp taste. Acids have a sour taste. Other acids are weak electrolytes that exist. Acids can be harmful and corrosive. A base is a substance that can.. What Are Some Properties Of An Acid.
From studylib.net
Properties of acids and bases What Are Some Properties Of An Acid Their ph values are always less than 7. Acids have a sour taste. When reacted with metals, these substances produce hydrogen gas. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. They are good conductors of electricity. — properties of acids. some acids are strong electrolytes because they ionize completely in water, yielding a great. What Are Some Properties Of An Acid.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry What Are Some Properties Of An Acid Lemons, vinegar, and sour candies all contain acids. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Their ph values are always less than 7. A base is a substance that can. — properties of acids. Acids can be harmful and corrosive. Acids. What Are Some Properties Of An Acid.
From www.worksheetsplanet.com
What is an Acid Definition of Acid What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Lemons, vinegar, and sour candies all contain acids. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Acids are sour in taste. A. What Are Some Properties Of An Acid.
From chemnotcheem.com
Definition and properties of acids O Level Chemistry Notes What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Acids have a sour taste. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. — properties of acids.. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Are Some Properties Of An Acid Other acids are weak electrolytes that exist. They are good conductors of electricity. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A dilute acid has a sour or sharp taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Sulfuric acid [h 2. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID What Are Some Properties Of An Acid Acids are sour in taste. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. Acids are corrosive in nature. Other acids are weak electrolytes that exist. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — properties of acids. When reacted with metals, these substances produce. What Are Some Properties Of An Acid.
From spmchemistry.blog.onlinetuition.com.my
Chemical Properties of Acids SPM Chemistry What Are Some Properties Of An Acid Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Their ph values are always less than 7. Lemons, vinegar, and sour candies all contain acids. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Acids are sour in taste. Vinegar, lemon. What Are Some Properties Of An Acid.
From www.online-sciences.com
Classification of Acids according to its strength (degree of ionization What Are Some Properties Of An Acid Acids are corrosive in nature. Acids are sour in taste. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. A base is a substance that can.. What Are Some Properties Of An Acid.
From aminghori.blogspot.com
Lesson Plan of Properties and Uses of Acids (Acids, Alkalies and Salts What Are Some Properties Of An Acid When reacted with metals, these substances produce hydrogen gas. Other acids are weak electrolytes that exist. Acids are corrosive in nature. A base is a substance that can. Acids have a sour taste. Their ph values are always less than 7. Acids can be harmful and corrosive. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Sulfuric. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download What Are Some Properties Of An Acid Vinegar, lemon juice, car batteries. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Lemons, vinegar, and sour candies all contain acids. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh].. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5349215 What Are Some Properties Of An Acid When reacted with metals, these substances produce hydrogen gas. Acids are sour in taste. Lemons, vinegar, and sour candies all contain acids. Acids are corrosive in nature. A base is a substance that can. They are good conductors of electricity. Acids have a sour taste. Acids can be harmful and corrosive. — acid, any substance that in water solution. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID What Are Some Properties Of An Acid A dilute acid has a sour or sharp taste. A base is a substance that can. Acids can be harmful and corrosive. — acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus. Vinegar, lemon juice, car batteries. Their ph values are always less than 7. When reacted with metals,. What Are Some Properties Of An Acid.
From general.chemistrysteps.com
Naming Acids and Bases Chemistry Steps What Are Some Properties Of An Acid — properties of acids. Vinegar, lemon juice, car batteries. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. Acids have a sour taste. A dilute acid. What Are Some Properties Of An Acid.
From acidbaseindicator.weebly.com
Properties of Acids and Bases What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Lemons, vinegar, and sour candies all contain acids. Acids can be harmful and corrosive. Other acids are weak electrolytes that exist. They are good conductors of electricity. Vinegar, lemon juice, car batteries. Aqueous solutions of acids are electrolytes, meaning that they conduct electrical. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free What Are Some Properties Of An Acid some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Lemons, vinegar, and sour candies all contain acids. Acids can be harmful and corrosive. you will learn what is an acid, the properties of acids and bases, the ph scale, difference between acid vs base, and amphoteric compounds. They are good conductors. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID1994749 What Are Some Properties Of An Acid Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. Their ph values are always less than 7. Acids are sour in taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. — acid, any substance that in water solution tastes sour, changes the colour of certain. What Are Some Properties Of An Acid.
From www.slideserve.com
PPT Explaining the Properties of Acids & Bases PowerPoint What Are Some Properties Of An Acid Lemons, vinegar, and sour candies all contain acids. When reacted with metals, these substances produce hydrogen gas. They are good conductors of electricity. A base is a substance that can. Sulfuric acid [h 2 so 4], hydrochloric acid [hcl], acetic acid [ch 3 cooh]. — acid, any substance that in water solution tastes sour, changes the colour of certain. What Are Some Properties Of An Acid.