Calorimetry Equation Delta H . revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Calorimetry is simply the measurement of heat. Call this function enthalpy, h, defined by: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. calculate the mass of the solution from its volume and density and calculate the temperature change of the. This is typically done under two distinct conditions. When we study energy changes in chemical reactions, the most. to find δh δ h for a reaction, measure qp q p. calorimetry at constant pressure. H = u + pv enthalpy enthalpy: Either at constant pressure in.
from www.youtube.com
calculate the mass of the solution from its volume and density and calculate the temperature change of the. Either at constant pressure in. Call this function enthalpy, h, defined by: When we study energy changes in chemical reactions, the most. H = u + pv enthalpy enthalpy: This is typically done under two distinct conditions. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. calorimetry at constant pressure.
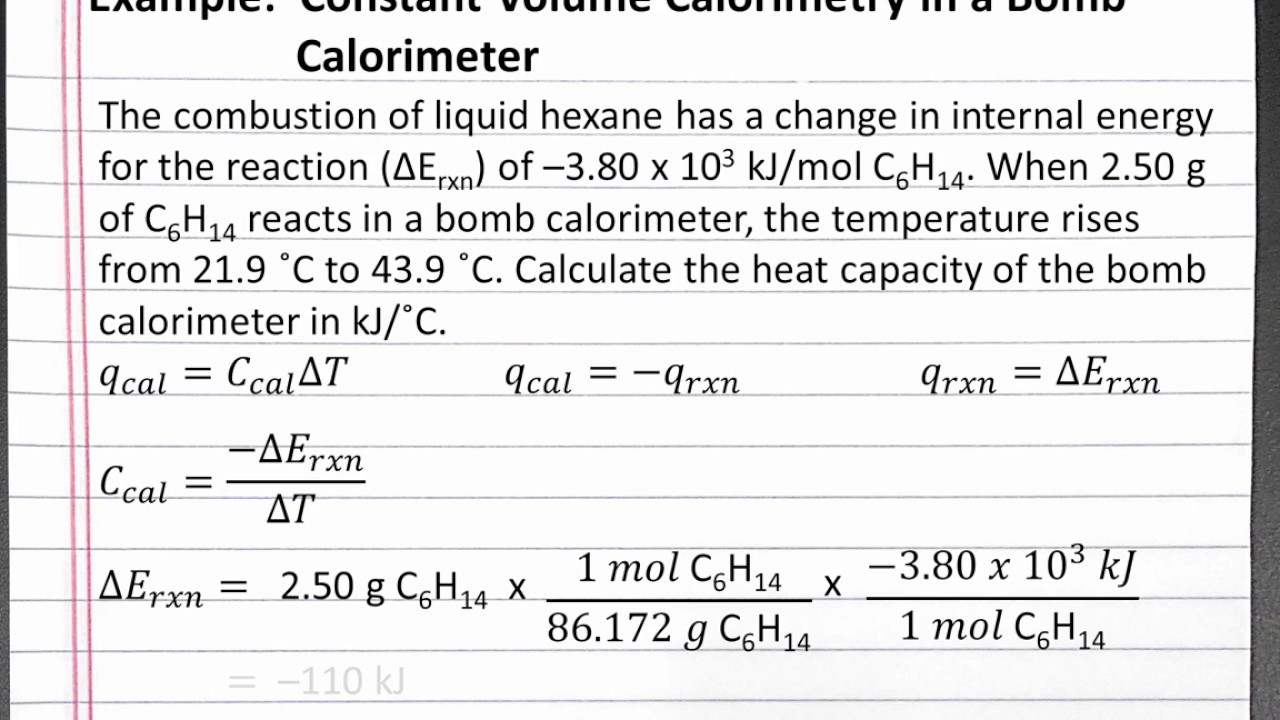
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
Calorimetry Equation Delta H Calorimetry is simply the measurement of heat. calorimetry at constant pressure. to find δh δ h for a reaction, measure qp q p. H = u + pv enthalpy enthalpy: Call this function enthalpy, h, defined by: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. calculate the mass of the solution from its volume and density and calculate the temperature change of the. When we study energy changes in chemical reactions, the most. Calorimetry is simply the measurement of heat. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. Either at constant pressure in. This is typically done under two distinct conditions. to calculate δh (change in enthalpy) using calorimetry, you can use the formula:
From allanfersmaxwell.blogspot.com
How to Calculate Delta H of Neutralization Calorimetry Equation Delta H Call this function enthalpy, h, defined by: Calorimetry is simply the measurement of heat. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. calculate the mass of the solution from its volume and density and calculate the temperature change of the. This is typically done under. Calorimetry Equation Delta H.
From www.youtube.com
Calorimetry, delta E and delta H YouTube Calorimetry Equation Delta H in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. to find δh δ h for a reaction, measure qp q p. to. Calorimetry Equation Delta H.
From www.youtube.com
Chemistry Help 5.11 Calculating Delta H using a Calorimetry Equation Delta H This is typically done under two distinct conditions. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: calculate the mass of the solution from its volume and density and calculate the temperature change of the. Either at constant pressure in. calorimetry at constant pressure. When we study energy changes in chemical reactions, the. Calorimetry Equation Delta H.
From www.youtube.com
Calorimetry & Enthalpy Problems YouTube Calorimetry Equation Delta H Either at constant pressure in. Call this function enthalpy, h, defined by: to find δh δ h for a reaction, measure qp q p. This is typically done under two distinct conditions. H = u + pv enthalpy enthalpy: calorimetry at constant pressure. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: . Calorimetry Equation Delta H.
From exydhmsea.blob.core.windows.net
Calorimeter Enthalpy Of Vaporization at John Martinez blog Calorimetry Equation Delta H Either at constant pressure in. Calorimetry is simply the measurement of heat. This is typically done under two distinct conditions. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: to find δh δ h for a reaction, measure qp q p. Call this function enthalpy, h, defined by: revision notes on 3.4.2 calorimetry. Calorimetry Equation Delta H.
From fyocdslfs.blob.core.windows.net
Calorimetry Explained at Edith Wallis blog Calorimetry Equation Delta H to find δh δ h for a reaction, measure qp q p. Either at constant pressure in. Call this function enthalpy, h, defined by: to calculate δh (change in enthalpy) using calorimetry, you can use the formula: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for. Calorimetry Equation Delta H.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Calorimetry Equation Delta H calculate the mass of the solution from its volume and density and calculate the temperature change of the. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. When we study energy changes in chemical reactions, the most. to find δh δ h for a reaction,. Calorimetry Equation Delta H.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry Equation Delta H calculate the mass of the solution from its volume and density and calculate the temperature change of the. Calorimetry is simply the measurement of heat. Call this function enthalpy, h, defined by: revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. calorimetry at constant pressure.. Calorimetry Equation Delta H.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Equation Delta H This is typically done under two distinct conditions. Call this function enthalpy, h, defined by: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. to find δh δ h for a reaction, measure qp q p. H = u + pv enthalpy enthalpy: revision. Calorimetry Equation Delta H.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Equation Delta H When we study energy changes in chemical reactions, the most. calculate the mass of the solution from its volume and density and calculate the temperature change of the. Call this function enthalpy, h, defined by: to find δh δ h for a reaction, measure qp q p. in a thermochemical equation, the enthalpy change of a reaction. Calorimetry Equation Delta H.
From www.youtube.com
Finding Delta H from Coffee Cup Calorimeter Data (LAB calculation Calorimetry Equation Delta H This is typically done under two distinct conditions. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Calorimetry is simply the measurement of heat. calculate the mass of the solution from its volume and density and calculate the temperature change of the. to find δh δ h for a reaction, measure qp q. Calorimetry Equation Delta H.
From www.chegg.com
Solved calculate the value of delta H°rxn for Calorimetry Equation Delta H calorimetry at constant pressure. Call this function enthalpy, h, defined by: to calculate δh (change in enthalpy) using calorimetry, you can use the formula: This is typically done under two distinct conditions. Either at constant pressure in. Calorimetry is simply the measurement of heat. H = u + pv enthalpy enthalpy: calculate the mass of the solution. Calorimetry Equation Delta H.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Equation Delta H to calculate δh (change in enthalpy) using calorimetry, you can use the formula: calorimetry at constant pressure. Call this function enthalpy, h, defined by: revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. This is typically done under two distinct conditions. calculate the mass. Calorimetry Equation Delta H.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Equation Delta H H = u + pv enthalpy enthalpy: to find δh δ h for a reaction, measure qp q p. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. This is typically done under two distinct conditions. to calculate δh (change in enthalpy) using calorimetry,. Calorimetry Equation Delta H.
From www.youtube.com
How do you measure DeltaH? calorimetry YouTube Calorimetry Equation Delta H This is typically done under two distinct conditions. to find δh δ h for a reaction, measure qp q p. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: in a. Calorimetry Equation Delta H.
From www.thesciencehive.co.uk
Energetics (GCSE) — the science hive Calorimetry Equation Delta H This is typically done under two distinct conditions. calorimetry at constant pressure. When we study energy changes in chemical reactions, the most. Either at constant pressure in. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. revision notes on 3.4.2 calorimetry for the ocr. Calorimetry Equation Delta H.
From abbyseniorib2022.blogspot.com
How to Determine H (Calorimetry) Calorimetry Equation Delta H H = u + pv enthalpy enthalpy: to calculate δh (change in enthalpy) using calorimetry, you can use the formula: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. Calorimetry is simply the measurement of heat. calorimetry at constant pressure. calculate the mass. Calorimetry Equation Delta H.
From exywsebyp.blob.core.windows.net
Calorimetry Equation Physics at Charles Marshall blog Calorimetry Equation Delta H This is typically done under two distinct conditions. to find δh δ h for a reaction, measure qp q p. calculate the mass of the solution from its volume and density and calculate the temperature change of the. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation. Calorimetry Equation Delta H.
From www.youtube.com
Delta H and Calorimetry YouTube Calorimetry Equation Delta H This is typically done under two distinct conditions. Call this function enthalpy, h, defined by: calculate the mass of the solution from its volume and density and calculate the temperature change of the. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. revision notes. Calorimetry Equation Delta H.
From www.youtube.com
09.05 Calorimetry YouTube Calorimetry Equation Delta H to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Calorimetry is simply the measurement of heat. calorimetry at constant pressure. to find δh δ h for a reaction, measure qp q p. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for. Calorimetry Equation Delta H.
From lessonfullsclerites.z13.web.core.windows.net
Coffee Cup Calorimetry Equation Calorimetry Equation Delta H Call this function enthalpy, h, defined by: calculate the mass of the solution from its volume and density and calculate the temperature change of the. Calorimetry is simply the measurement of heat. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. When we study energy. Calorimetry Equation Delta H.
From ambrosiabaking.com
Coffee Cup Calorimeter A Simple And Inexpensive Way To Measure The Calorimetry Equation Delta H calculate the mass of the solution from its volume and density and calculate the temperature change of the. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Calorimetry is simply the measurement of heat. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save. Calorimetry Equation Delta H.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Equation Delta H Either at constant pressure in. H = u + pv enthalpy enthalpy: to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Call this function enthalpy, h, defined by: calculate the mass of the solution from its volume and density and calculate the temperature change of the. When we study energy changes in chemical reactions,. Calorimetry Equation Delta H.
From gioxxobze.blob.core.windows.net
Calorimetry Formula Reaction at Mazie Miller blog Calorimetry Equation Delta H H = u + pv enthalpy enthalpy: Calorimetry is simply the measurement of heat. calculate the mass of the solution from its volume and density and calculate the temperature change of the. Either at constant pressure in. Call this function enthalpy, h, defined by: This is typically done under two distinct conditions. to calculate δh (change in enthalpy). Calorimetry Equation Delta H.
From www.youtube.com
Class 11 Chemistry Measurement of Delta U and Delta H CALORIMETRY in Calorimetry Equation Delta H to calculate δh (change in enthalpy) using calorimetry, you can use the formula: calorimetry at constant pressure. H = u + pv enthalpy enthalpy: in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. Either at constant pressure in. When we study energy changes in. Calorimetry Equation Delta H.
From ar.inspiredpencil.com
Calorimetry Equation Calorimetry Equation Delta H When we study energy changes in chemical reactions, the most. Call this function enthalpy, h, defined by: This is typically done under two distinct conditions. H = u + pv enthalpy enthalpy: Calorimetry is simply the measurement of heat. calorimetry at constant pressure. to find δh δ h for a reaction, measure qp q p. revision notes. Calorimetry Equation Delta H.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Equation Delta H H = u + pv enthalpy enthalpy: Either at constant pressure in. Calorimetry is simply the measurement of heat. This is typically done under two distinct conditions. calculate the mass of the solution from its volume and density and calculate the temperature change of the. to calculate δh (change in enthalpy) using calorimetry, you can use the formula:. Calorimetry Equation Delta H.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimetry Equation Delta H H = u + pv enthalpy enthalpy: calorimetry at constant pressure. Call this function enthalpy, h, defined by: revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. Calorimetry is simply the measurement of heat. When we study energy changes in chemical reactions, the most. in. Calorimetry Equation Delta H.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Equation Delta H to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Call this function enthalpy, h, defined by: Calorimetry is simply the measurement of heat. Either at constant pressure in. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. This is typically done under. Calorimetry Equation Delta H.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Equation Delta H When we study energy changes in chemical reactions, the most. Calorimetry is simply the measurement of heat. calculate the mass of the solution from its volume and density and calculate the temperature change of the. H = u + pv enthalpy enthalpy: calorimetry at constant pressure. Call this function enthalpy, h, defined by: in a thermochemical equation,. Calorimetry Equation Delta H.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Calorimetry Equation Delta H in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. calorimetry at constant pressure. When we study energy changes in chemical reactions, the most. to find δh δ h for a reaction, measure qp q p. calculate the mass of the solution from its. Calorimetry Equation Delta H.
From www.chegg.com
Solved Given the following data Calculate delta H^degree Calorimetry Equation Delta H to find δh δ h for a reaction, measure qp q p. Call this function enthalpy, h, defined by: Either at constant pressure in. When we study energy changes in chemical reactions, the most. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. to. Calorimetry Equation Delta H.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation Delta H Call this function enthalpy, h, defined by: When we study energy changes in chemical reactions, the most. revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: calorimetry at constant pressure. Either at. Calorimetry Equation Delta H.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Calorimetry Equation Delta H in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction. to calculate δh (change in enthalpy) using calorimetry, you can use the formula: Either at constant pressure in. When we study energy changes in chemical reactions, the most. calorimetry at constant pressure. calculate the. Calorimetry Equation Delta H.
From www.youtube.com
Heat and Enthalpy q and delta H YouTube Calorimetry Equation Delta H revision notes on 3.4.2 calorimetry for the ocr a level chemistry syllabus, written by the chemistry experts at save my exams. Either at constant pressure in. When we study energy changes in chemical reactions, the most. in a thermochemical equation, the enthalpy change of a reaction is shown as a δh value following the equation for the reaction.. Calorimetry Equation Delta H.