Example Of Gmp Certificate . Chemical and chemical product manufacturing. This is not part of. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Animal health and veterinary product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to.
from www.uaeiso.org
Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. medical device manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. This is not part of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Animal health and veterinary product manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Chemical and chemical product manufacturing.
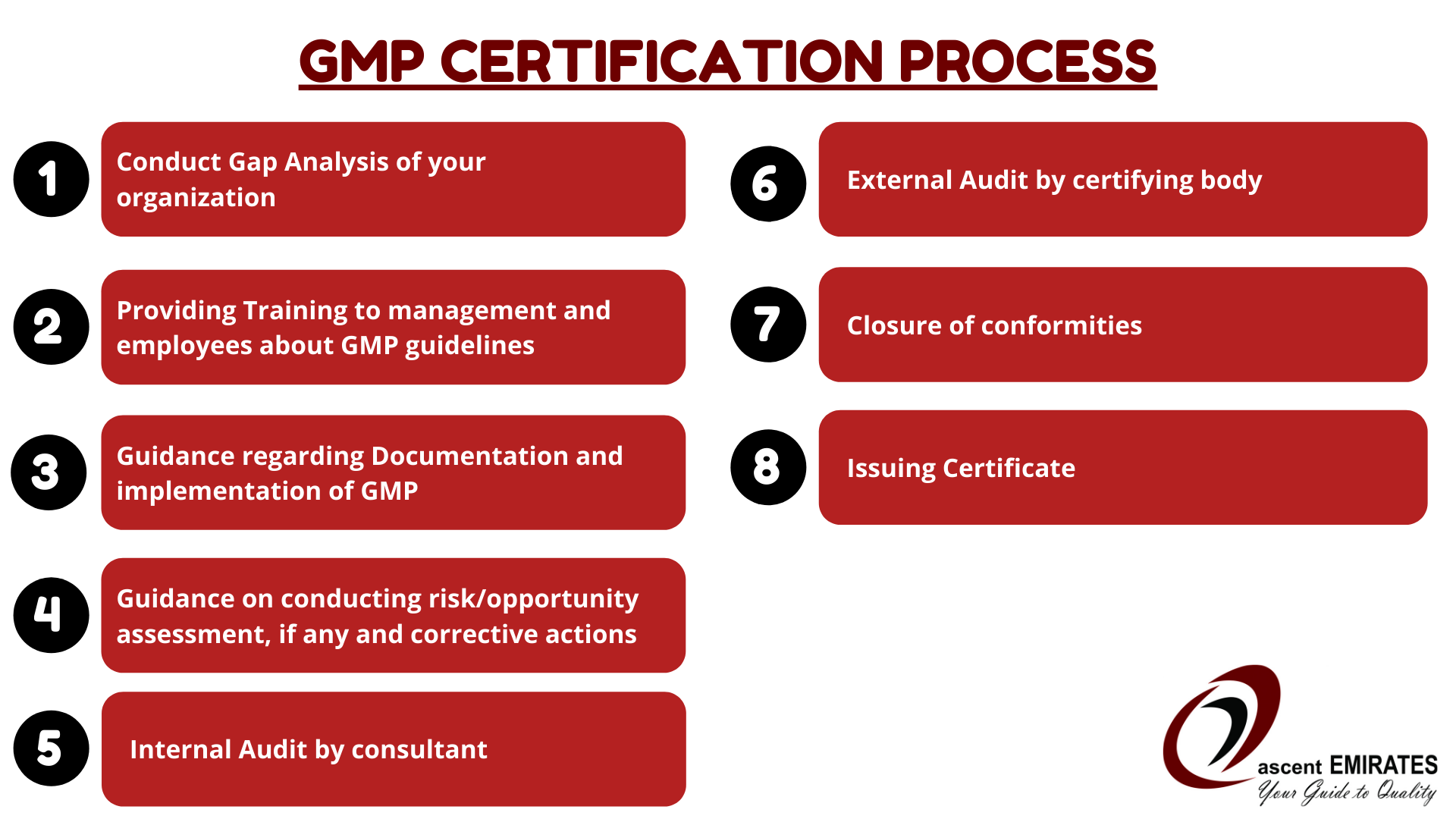
GMP Certification Check Benefits, Safety & Integrity Ascent
Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. medical device manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Animal health and veterinary product manufacturing. Chemical and chemical product manufacturing. This is not part of. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in.
From co.ap-rad.com
GMP Certificate AmirPayvand Research & Development Company Example Of Gmp Certificate good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Chemical and chemical product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. This is. Example Of Gmp Certificate.
From www.foodtechproducts.com
gmp certificate Example Of Gmp Certificate This is not part of. Chemical and chemical product manufacturing. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Animal health and veterinary product manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. in july 2010 documents connected with good distribution practice (gdp). Example Of Gmp Certificate.
From veerhealthcare.net
GMP Certificate Veer Health Care Example Of Gmp Certificate Chemical and chemical product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to.. Example Of Gmp Certificate.
From www.trickymag.com
The Definitive Guide to GMP Certificate Tricky Mag Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. a model certificate of good. Example Of Gmp Certificate.
From www.modelgroupbd.com
GMP,ISO and USFDA CERTIFICATE Example Of Gmp Certificate Chemical and chemical product manufacturing. Animal health and veterinary product manufacturing. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect.. Example Of Gmp Certificate.
From www.arogyafarm.lk
GMP Certificate Arogya Farm Example Of Gmp Certificate Animal health and veterinary product manufacturing. medical device manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. . Example Of Gmp Certificate.
From tutore.org
Who Gmp Certificate Renewal Master of Documents Example Of Gmp Certificate medical device manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). This. Example Of Gmp Certificate.
From www.hsbiotech.com
TUV SUD Codex GMP Certificate Certificates HSIEHS BIOTECH Example Of Gmp Certificate Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Animal health and veterinary product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). good manufacturing practice. Example Of Gmp Certificate.
From www.newa1.com
Certification A1 Family Example Of Gmp Certificate Animal health and veterinary product manufacturing. This is not part of. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. good manufacturing practice (gmp) describes the minimum standard that a. Example Of Gmp Certificate.
From germisep.com
GMP Certification Germisep Example Of Gmp Certificate good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. The purpose of this document is to provide guidance to industry. Example Of Gmp Certificate.
From www.gmchemie.com
Principles of GMP Policy, G. M. P. Certificate, FDA Licence, G. M Example Of Gmp Certificate Animal health and veterinary product manufacturing. This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. medical device manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections. Example Of Gmp Certificate.
From vaadiorganics.mu
GMP Certified — Vaadi Organics Mauritius Ayurveda with mode... Example Of Gmp Certificate medical device manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. This is not part of. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Chemical and chemical product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site. Example Of Gmp Certificate.
From www.gognathexim.com
GMP Certificate Example Of Gmp Certificate a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). Chemical and chemical product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Animal health and veterinary product manufacturing. medical device manufacturing. good manufacturing practice (gmp) describes the minimum standard that a. Example Of Gmp Certificate.
From www.sirona-naturals.com
GMP Certified Manufacturing & Quality Assurance Safe For Consumption. Example Of Gmp Certificate The purpose of this document is to provide guidance to industry and regulators on the interpretation of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). Chemical and chemical product manufacturing. Animal health and veterinary product manufacturing. This is not part of. good manufacturing practice (gmp) describes the minimum standard that. Example Of Gmp Certificate.
From mavink.com
Good Manufacturing Practices Gmp Certificate Example Of Gmp Certificate Animal health and veterinary product manufacturing. Chemical and chemical product manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect.. Example Of Gmp Certificate.
From www.uaeiso.org
GMP Certification Check Benefits, Safety & Integrity Ascent Example Of Gmp Certificate This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. The purpose of this document is to provide guidance to industry and regulators on the interpretation of.. Example Of Gmp Certificate.
From www.taimacn.com
GMP Certificate Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. medical device manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Animal. Example Of Gmp Certificate.
From www.ichn.gliwice.pl
GMP certificate for Chemistry Division "IChN" as a Example Of Gmp Certificate medical device manufacturing. Animal health and veterinary product manufacturing. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. This is not part of. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. a model certificate of good manufacturing practices (gmp) for a. Example Of Gmp Certificate.
From headwaysz.en.made-in-china.com
GMP Certificate Shenzhen Zhonghe Headway BioSci & Tech Co., Ltd. Example Of Gmp Certificate The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Chemical and chemical product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. This is not part of. Animal health and veterinary product manufacturing. Gmp certificates are for the purpose of confirming the overall. Example Of Gmp Certificate.
From jiangnancn.en.hisupplier.com
GMP Certificate Zhejiang Jiangnan Pharmaceutical Machinery Co., Ltd. Example Of Gmp Certificate medical device manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. This is not part of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). Animal health and veterinary product manufacturing. good manufacturing practice (gmp) describes. Example Of Gmp Certificate.
From www.fodder-yeast.com
GMP+ Certificate B&B Feed and yeast Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. medical device manufacturing. Animal health and veterinary product manufacturing. This. Example Of Gmp Certificate.
From www.pinterest.com
sample gmp certificate template doc in 2021 Certificate templates Example Of Gmp Certificate Animal health and veterinary product manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Chemical and chemical product. Example Of Gmp Certificate.
From www.ncs-science.com
GMP Certificate (2)1 NCS Science Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. This is not part of. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). The purpose of this document. Example Of Gmp Certificate.
From quartz.pl
GMP Good Manufacturing Practice Certificates Example Of Gmp Certificate This is not part of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Animal health and veterinary product manufacturing. Chemical and chemical product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. good manufacturing practice (gmp) describes the minimum standard that a. Example Of Gmp Certificate.
From dhti.vn
What is a GMP certificate? Steps to a GMPcertified Example Of Gmp Certificate Chemical and chemical product manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. medical device manufacturing. This is not part of. The purpose of this document is to provide guidance to industry and regulators on. Example Of Gmp Certificate.
From www.slideshare.net
GMP Certificate Example Of Gmp Certificate a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. This is not part of. medical device manufacturing. . Example Of Gmp Certificate.
From www.acrossbarriers.de
GLP / GMP / FDA Across Barriers GmbH Example Of Gmp Certificate a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. . Example Of Gmp Certificate.
From tutore.org
Who Gmp Certificate Format Master of Documents Example Of Gmp Certificate Animal health and veterinary product manufacturing. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Chemical and chemical product manufacturing.. Example Of Gmp Certificate.
From www.qimingvc.com
CanSinoBIO Receives EU GMP Certificate Company Qiming Venture Partners Example Of Gmp Certificate Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Animal health and veterinary product manufacturing. Chemical and chemical product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. The purpose of this document is to provide guidance to industry and regulators on the interpretation. Example Of Gmp Certificate.
From www.vrogue.co
Sample Gmp Certificate Template Doc In 2021 Certifica vrogue.co Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). The purpose of this document is to provide guidance to industry and regulators on the interpretation of. Gmp certificates are for the purpose of confirming the. Example Of Gmp Certificate.
From www.biosyyd.com
EU GMP (Pharmaceutical) Certification — Biosyyd Example Of Gmp Certificate This is not part of. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. Chemical and chemical product manufacturing. Animal health and veterinary product manufacturing. good manufacturing practice (gmp) describes the. Example Of Gmp Certificate.
From www.hsbiotech.com
TUV SUD Codex GMP Certificate Certificates HSIEHS BIOTECH Example Of Gmp Certificate in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). Chemical and chemical product manufacturing. This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in.. Example Of Gmp Certificate.
From www.abrodex.com
[GMP Certificate Attestation Malaysia Embassy] GMP Legalization Example Of Gmp Certificate Animal health and veterinary product manufacturing. Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). medical device manufacturing. This is not part of. good manufacturing practice (gmp) describes the minimum standard that a medicines. Example Of Gmp Certificate.
From www.gmchemie.com
Principles of GMP Policy, G. M. P. Certificate, FDA Licence, G. M Example Of Gmp Certificate medical device manufacturing. a model certificate of good manufacturing practices (gmp) for a manufacturing site is suggested (see below). good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. The purpose of this document is to provide guidance to industry and regulators on the interpretation of. This is not part of. . Example Of Gmp Certificate.
From athenaoem.en.made-in-china.com
GMP Certificate Athena (Guangzhou) Cosmetics Manufacturer Co., Ltd. Example Of Gmp Certificate Gmp certificates are for the purpose of confirming the overall conclusion of an inspection with respect. Animal health and veterinary product manufacturing. in july 2010 documents connected with good distribution practice (gdp) inspections started to be added to. Chemical and chemical product manufacturing. This is not part of. good manufacturing practice (gmp) describes the minimum standard that a. Example Of Gmp Certificate.