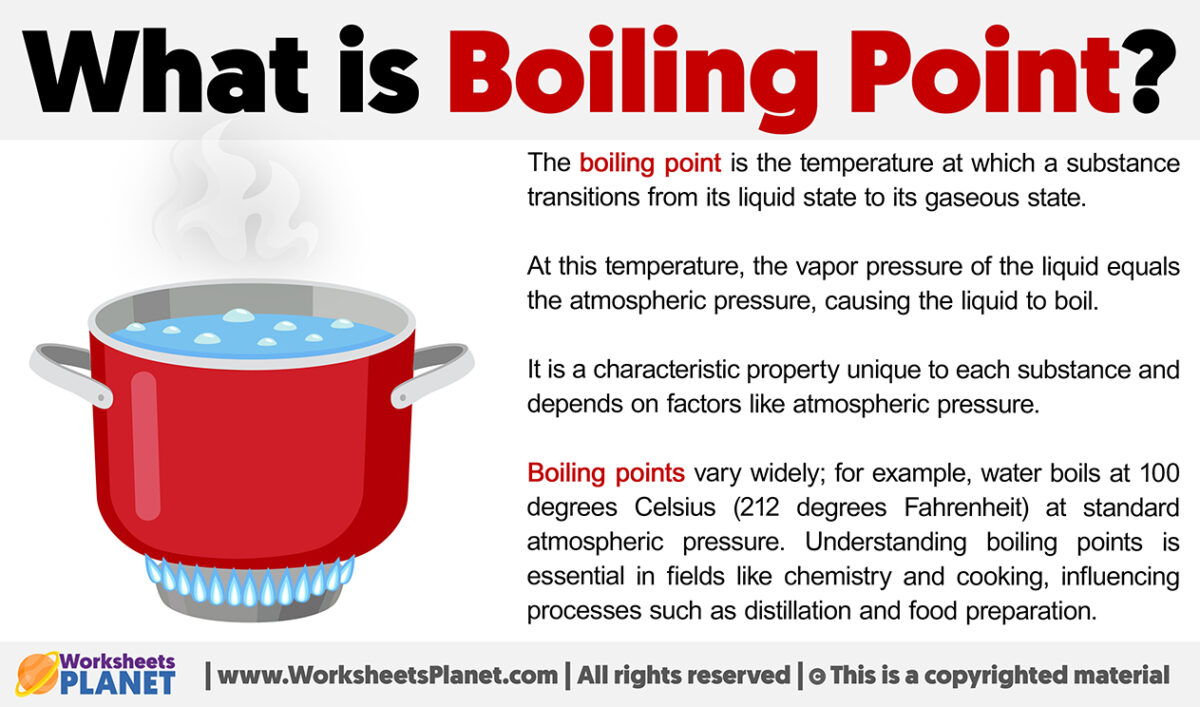
What Is A Normal Boiling Point . the boiling point of a liquid varies according to the applied pressure; a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The normal boiling point is the temperature at which the vapour. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure.
from www.worksheetsplanet.com
the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The “normal” refers to sea level or an elevation of 0 meters or feet. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the boiling point of a liquid varies according to the applied pressure; the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The normal boiling point is the temperature at which the vapour.
What is Boiling Point
What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The “normal” refers to sea level or an elevation of 0 meters or feet. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \:
From www.chegg.com
Solved What is the normal boiling point of the substance What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: the. What Is A Normal Boiling Point.
From srkxqssnsldfz.blogspot.com
How To Calculate Normal Boiling Point Number of i groups in molecule What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the boiling point of a liquid varies according to the applied pressure; the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. the normal boiling point is the temperature at which the vapor pressure of the. What Is A Normal Boiling Point.
From schematiclistsalem123.z22.web.core.windows.net
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point is the temperature at which the vapor pressure of the. What Is A Normal Boiling Point.
From wirepartfrontwards.z14.web.core.windows.net
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. What Is A Normal Boiling Point.
From gioulmxcr.blob.core.windows.net
What Is The Boiling Point Of A Cat at Anthony Dean blog What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: the boiling point of a liquid varies according to the applied pressure; if this pressure is the standard pressure of. What Is A Normal Boiling Point.
From www.chegg.com
Solved What is the normal boiling point in ∘C for B (Orange) What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal boiling point is the. What Is A Normal Boiling Point.
From www.youtube.com
Normal boiling point `(T_(N))` is defined as the temperature when V.P What Is A Normal Boiling Point the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The normal boiling point is the temperature at which the vapour. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. normal boiling point is. What Is A Normal Boiling Point.
From drivenheisenberg.blogspot.com
The Normal Boiling Point For The Substance In The Phase Diagram Below What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The normal boiling point is. What Is A Normal Boiling Point.
From gamesmartz.com
Normal Boiling Point Definition & Image GameSmartz What Is A Normal Boiling Point if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of. What Is A Normal Boiling Point.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation What Is A Normal Boiling Point The “normal” refers to sea level or an elevation of 0 meters or feet. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: the boiling point of a liquid varies according to the applied pressure; normal boiling point is the temperature at which a liquid boils at 1 atmosphere of. What Is A Normal Boiling Point.
From www.slideserve.com
PPT Gas Pressure PowerPoint Presentation, free download ID1445532 What Is A Normal Boiling Point if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point is the temperature at which the vapour. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: the normal. What Is A Normal Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Normal Boiling Point The “normal” refers to sea level or an elevation of 0 meters or feet. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \:. What Is A Normal Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is A Normal Boiling Point a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The normal boiling point is the temperature at which the vapour. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal. What Is A Normal Boiling Point.
From www.youtube.com
CHEMISTRY 201 Calculating Boiling Point Using Clausius Clapeyron What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The “normal” refers to sea level or an elevation of 0 meters or feet.. What Is A Normal Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point What Is A Normal Boiling Point The normal boiling point is the temperature at which the vapour. the boiling point of a liquid varies according to the applied pressure; the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils. What Is A Normal Boiling Point.
From guidemanualeruptivity.z14.web.core.windows.net
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. What Is A Normal Boiling Point.
From www.toppr.com
The normal boiling point of water is 373 K . V What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. the normal boiling point is the temperature at which the vapor pressure of the. What Is A Normal Boiling Point.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is A Normal Boiling Point a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The “normal” refers to sea level or an elevation of 0 meters or feet. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point.. What Is A Normal Boiling Point.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID5814893 What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal boiling point is the temperature at which the vapor pressure of. What Is A Normal Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Normal Boiling Point normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. What Is A Normal Boiling Point.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is A Normal Boiling Point a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The normal boiling point is the temperature at which the vapour. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. if this pressure is the standard pressure of 1 atm. What Is A Normal Boiling Point.
From jumpstarterdiscount.blogspot.com
Normal Boiling Point On Phase Diagram Wiring Diagram What Is A Normal Boiling Point The “normal” refers to sea level or an elevation of 0 meters or feet. The normal boiling point is the temperature at which the vapour. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the normal boiling point is. What Is A Normal Boiling Point.
From www.difference.wiki
Normal Boiling Point vs. Standard Boiling Point What’s the Difference? What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. . What Is A Normal Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Normal Boiling Point if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point is the temperature at which the vapour. the boiling point of a liquid varies according to the applied pressure; the normal boiling point is the. What Is A Normal Boiling Point.
From jumpstarterdiscount.blogspot.com
Normal Boiling Point On Phase Diagram Wiring Diagram What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The “normal” refers to. What Is A Normal Boiling Point.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the What Is A Normal Boiling Point The normal boiling point is the temperature at which the vapour. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: the normal. What Is A Normal Boiling Point.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID596601 What Is A Normal Boiling Point The normal boiling point is the temperature at which the vapour. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The “normal” refers to sea level or an elevation of 0 meters or feet. if this pressure is the standard pressure of 1 atm (101.3 kpa), then. What Is A Normal Boiling Point.
From manuallistcoverslip.z1.web.core.windows.net
Normal Boiling Point On Phase Diagram What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The “normal” refers to sea level or an elevation of 0 meters or feet. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The normal boiling point is the temperature at which the. What Is A Normal Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. the boiling point of a liquid varies according to the applied pressure; The “normal” refers to sea level or an elevation of 0 meters or feet. normal boiling point is the temperature at which a liquid boils. What Is A Normal Boiling Point.
From wiringliststaphyloma.z14.web.core.windows.net
How To Calculate The Normal Boiling Point What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. a compound's normal boiling point refers to its boiling point. What Is A Normal Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. a compound's normal boiling point refers to its boiling point at a pressure of \(760 \: The normal boiling point is the temperature at which. What Is A Normal Boiling Point.
From wiringdbnightwear.z21.web.core.windows.net
Normal Boiling Point On Phase Diagram What Is A Normal Boiling Point the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The normal boiling point is the temperature at which the vapour. the normal boiling point of water is 100 °c, 212. What Is A Normal Boiling Point.
From mavink.com
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. if this pressure is the standard pressure of 1. What Is A Normal Boiling Point.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is A Normal Boiling Point the boiling point of a liquid varies according to the applied pressure; normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The normal boiling point is the temperature at which. What Is A Normal Boiling Point.
From schematicdiagramtelium.z13.web.core.windows.net
How To Determine Normal Boiling Point What Is A Normal Boiling Point The normal boiling point is the temperature at which the vapour. the normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The “normal” refers to sea level or an elevation of 0 meters or feet. if this pressure is the standard pressure of 1 atm (101.3 kpa), then. What Is A Normal Boiling Point.