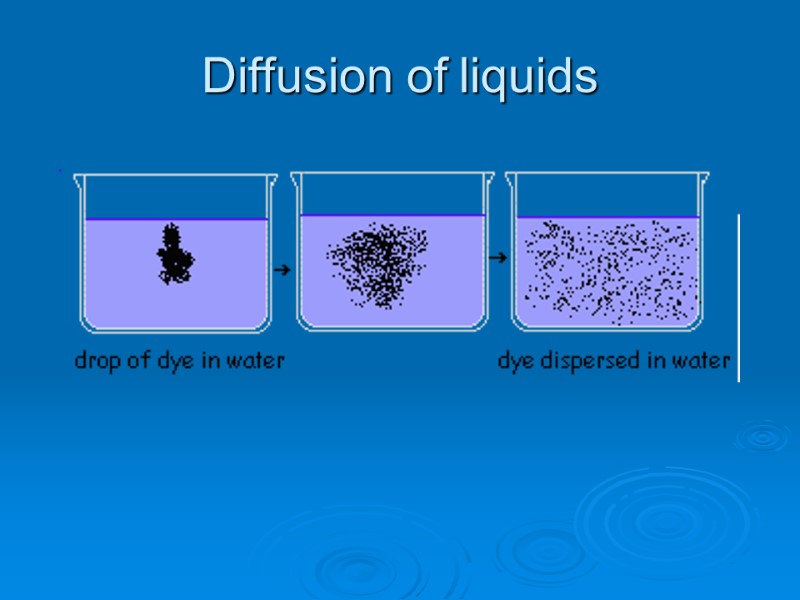
What Is Diffusion Of Solids In Liquids . When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Find out when it occurs, its types and characteristics explained with examples and. There must be an empty adjacent site. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Jelly is a liquid before it has set and looks like a solid when it has set. At equilibrium, the net effect is a. Diffusion occurs because gas and liquid particles move at. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Learn what is diffusion and what factors affect it.
from present5.com
At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Jelly is a liquid before it has set and looks like a solid when it has set. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion occurs because gas and liquid particles move at. There must be an empty adjacent site. Learn what is diffusion and what factors affect it. At equilibrium, the net effect is a. Find out when it occurs, its types and characteristics explained with examples and.
The Transport of Materials Across the Cell membrane
What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Jelly is a liquid before it has set and looks like a solid when it has set. Learn what is diffusion and what factors affect it. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. There must be an empty adjacent site. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion occurs because gas and liquid particles move at. At equilibrium, the net effect is a. Find out when it occurs, its types and characteristics explained with examples and.
From www.youtube.com
Diffusion class 9 Diffusion in solids Diffusion in liquids What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. Find out when it occurs, its types and characteristics explained with examples and. At equilibrium, the net effect is a. Learn what is diffusion and what factors affect it. Diffusion occurs because gas and liquid particles move at. At any temperature different from. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT The Plasma Membrane PowerPoint Presentation, free download ID What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. At equilibrium, the net effect is a. There must be an empty adjacent site. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Find out when it occurs, its types and characteristics. What Is Diffusion Of Solids In Liquids.
From www.shutterstock.com
Diffusion solids liquids Images, Stock Photos & Vectors Shutterstock What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Find out when it occurs, its types and characteristics explained with examples and. Learn what is diffusion and what factors affect it. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion. What Is Diffusion Of Solids In Liquids.
From www.visionlearning.com
Diffusion I Chemistry Visionlearning What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. Learn what is diffusion and what factors affect it. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Find out when it occurs, its types and characteristics explained with. What Is Diffusion Of Solids In Liquids.
From www.thoughtco.com
Examples of Diffusion in Chemistry What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion occurs because gas and liquid particles move at. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At equilibrium, the net effect is a. Learn what is diffusion and what factors. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Behavior of Liquids and Gases Diffusion and Pressure PowerPoint What Is Diffusion Of Solids In Liquids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At equilibrium, the net effect is a. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Jelly is a liquid before it has set and looks like a solid when it has. What Is Diffusion Of Solids In Liquids.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science What Is Diffusion Of Solids In Liquids Find out when it occurs, its types and characteristics explained with examples and. There must be an empty adjacent site. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Jelly is a liquid before it has set and looks like a solid when it has set.. What Is Diffusion Of Solids In Liquids.
From www.sciencefacts.net
Diffusion Definition and How Does it Occur (with Diagram) What Is Diffusion Of Solids In Liquids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At equilibrium, the net effect is a. There must be an empty adjacent site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Jelly is a liquid before it has set and. What Is Diffusion Of Solids In Liquids.
From www.youtube.com
Diffusion in Solid Chemistry YouTube What Is Diffusion Of Solids In Liquids Find out when it occurs, its types and characteristics explained with examples and. At equilibrium, the net effect is a. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. When a substance spreads out from an area where there are a lot of particles to an. What Is Diffusion Of Solids In Liquids.
From www.studypool.com
SOLUTION Diffusion in solids liquids and gases Studypool What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion occurs because gas and liquid particles move at. Diffusion is just a stepwise migration of atoms from lattice site. What Is Diffusion Of Solids In Liquids.
From www.scribd.com
To Study the Diffusion of Solids in Liquids Diffusion Particle What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. There must be an empty adjacent site. Find out when it occurs, its types and characteristics explained with examples and.. What Is Diffusion Of Solids In Liquids.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry What Is Diffusion Of Solids In Liquids Diffusion occurs because gas and liquid particles move at. Jelly is a liquid before it has set and looks like a solid when it has set. At equilibrium, the net effect is a. Find out when it occurs, its types and characteristics explained with examples and. When a substance spreads out from an area where there are a lot of. What Is Diffusion Of Solids In Liquids.
From brainly.in
Illustrate an experiment how diffusion in liquid can be demonstrated What Is Diffusion Of Solids In Liquids At equilibrium, the net effect is a. There must be an empty adjacent site. Diffusion occurs because gas and liquid particles move at. Jelly is a liquid before it has set and looks like a solid when it has set. Find out when it occurs, its types and characteristics explained with examples and. Learn what is diffusion and what factors. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Solids, liquids and gases PowerPoint Presentation, free download What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. Find out when it occurs, its types and characteristics explained with examples and. At equilibrium, the net effect is a. Learn what is diffusion and what factors affect it. When a substance spreads out from an area where there are a lot of. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of What Is Diffusion Of Solids In Liquids There must be an empty adjacent site. Learn what is diffusion and what factors affect it. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. When a substance spreads out from an area where there are a lot of particles to an area where there are. What Is Diffusion Of Solids In Liquids.
From www.visionlearning.com
Visionlearning Chemistry Diffusion I What Is Diffusion Of Solids In Liquids Diffusion occurs because gas and liquid particles move at. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Jelly is a liquid before it has set and looks like a solid when it has set. At equilibrium, the net effect is a. Learn what is diffusion. What Is Diffusion Of Solids In Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo What Is Diffusion Of Solids In Liquids Diffusion occurs because gas and liquid particles move at. At equilibrium, the net effect is a. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. There must be an empty adjacent site.. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Water and its relation to Diffusion and Osmosis PowerPoint What Is Diffusion Of Solids In Liquids Find out when it occurs, its types and characteristics explained with examples and. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. At equilibrium, the net effect is a. Learn what is diffusion and what factors affect it. Diffusion is just a stepwise migration of atoms. What Is Diffusion Of Solids In Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo What Is Diffusion Of Solids In Liquids When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Find out when it occurs, its types and characteristics explained with examples and. At equilibrium, the net effect is a. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6716640 What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. Find out when it occurs, its types and characteristics explained with examples and. There must be an empty adjacent site. Learn what is diffusion and what factors affect it. When a substance spreads out from an area where there are a lot of. What Is Diffusion Of Solids In Liquids.
From www.shalom-education.com
Diffusion GCSE Biology Revision What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Find out when it occurs, its types and characteristics explained with examples and. Learn what is diffusion and what factors affect it. When. What Is Diffusion Of Solids In Liquids.
From present5.com
The Transport of Materials Across the Cell membrane What Is Diffusion Of Solids In Liquids Jelly is a liquid before it has set and looks like a solid when it has set. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion occurs because gas and liquid particles move at. Diffusion is just a stepwise migration of atoms from lattice site. What Is Diffusion Of Solids In Liquids.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Is Diffusion Of Solids In Liquids Find out when it occurs, its types and characteristics explained with examples and. Diffusion occurs because gas and liquid particles move at. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. At equilibrium, the net effect is a. Learn what is diffusion and what factors affect. What Is Diffusion Of Solids In Liquids.
From thescienceteacher.co.uk
Diffusion teaching resources the science teacher What Is Diffusion Of Solids In Liquids At equilibrium, the net effect is a. Jelly is a liquid before it has set and looks like a solid when it has set. Diffusion occurs because gas and liquid particles move at. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. There must be an. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion Of Solids In Liquids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. There must be an empty adjacent site. Find out when it occurs, its types and characteristics explained with examples and. At equilibrium, the. What Is Diffusion Of Solids In Liquids.
From www.jove.com
11340.jpg What Is Diffusion Of Solids In Liquids Learn what is diffusion and what factors affect it. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Diffusion occurs because gas and liquid particles move at. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid),. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT Lecture 3a. PowerPoint Presentation, free download ID814388 What Is Diffusion Of Solids In Liquids At equilibrium, the net effect is a. Find out when it occurs, its types and characteristics explained with examples and. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. There must be an empty adjacent site. Learn what is diffusion and what factors affect it. Diffusion. What Is Diffusion Of Solids In Liquids.
From www.slideshare.net
solids, liquids and gases What Is Diffusion Of Solids In Liquids When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. There must be an empty adjacent site. Learn what is diffusion and what factors affect it. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly. What Is Diffusion Of Solids In Liquids.
From eduinput.com
Why is Diffusion Faster in Liquids than Solids? What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Find out when it occurs, its types and characteristics explained with examples and. Diffusion occurs because gas and liquid particles move at. When. What Is Diffusion Of Solids In Liquids.
From byjus.com
What is diffusion? Explain about diffusion in solid , liquid and gas What Is Diffusion Of Solids In Liquids Diffusion occurs because gas and liquid particles move at. Jelly is a liquid before it has set and looks like a solid when it has set. When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Learn what is diffusion and what factors affect it. Find out. What Is Diffusion Of Solids In Liquids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Find out when it occurs, its types and characteristics explained with examples and. Learn what is diffusion and what factors affect it. Diffusion occurs because gas and liquid particles move at. When a substance spreads out from. What Is Diffusion Of Solids In Liquids.
From nayturr.com
3 Types of Diffusion (Plus Examples for Each) Nayturr What Is Diffusion Of Solids In Liquids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Find out when it occurs, its types and characteristics explained with examples and. Diffusion occurs because gas and liquid particles move at. Jelly is a liquid before it has set and looks like a solid when it has set. When a substance spreads out from an. What Is Diffusion Of Solids In Liquids.
From byjus.com
Explain the diffusion in liquids with two examples. What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Find out when it occurs, its types and characteristics explained with examples and. Diffusion occurs because gas and liquid particles move at. There must be an empty adjacent site. At equilibrium, the net effect is a. Jelly. What Is Diffusion Of Solids In Liquids.
From mungfali.com
Solids Liquids Gases Chart What Is Diffusion Of Solids In Liquids When a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. There must be an empty adjacent site. Learn what is diffusion and what factors affect it. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion occurs because gas and liquid particles. What Is Diffusion Of Solids In Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo What Is Diffusion Of Solids In Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion occurs because gas and liquid particles move at. Learn what is diffusion and what factors affect it. At equilibrium, the net effect is a. Jelly is a liquid before it has set and looks like a. What Is Diffusion Of Solids In Liquids.