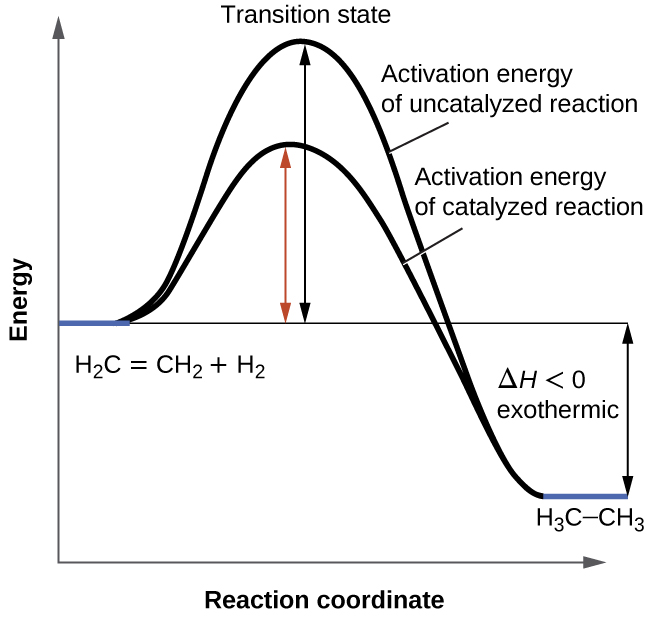
Why Do Catalysts Not Get Used Up . Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. When a catalyst is added, it is not used up or chemically changed in the reaction. Catalysts are substances that speed up a reaction but which are not. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page explains how adding a catalyst affects the rate of a reaction. We can prove this by weighing our catalyst at the beginning. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate.
from psu.pb.unizin.org
We can prove this by weighing our catalyst at the beginning. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. They do not appear in the reaction’s net equation and are not consumed during the reaction. When the reaction has finished, you would have. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. Catalysts participate in a chemical reaction and increase its rate. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts allow a reaction to.
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General
Why Do Catalysts Not Get Used Up When the reaction has finished, you would have. Catalysts are substances that speed up a reaction but which are not. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This page explains how adding a catalyst affects the rate of a reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. Catalysts participate in a chemical reaction and increase its rate. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. When a catalyst is added, it is not used up or chemically changed in the reaction. We can prove this by weighing our catalyst at the beginning. Catalysts allow a reaction to. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell.
From slideplayer.com
Ch. 6 Biochemistry Questions of the Day ppt download Why Do Catalysts Not Get Used Up Catalysts participate in a chemical reaction and increase its rate. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. We can prove this by weighing our catalyst at the beginning. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page explains how adding a catalyst affects the. Why Do Catalysts Not Get Used Up.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. When the reaction has finished,. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Why Do Catalysts Not Get Used Up We can prove this by weighing our catalyst at the beginning. When a catalyst is added, it is not used up or chemically changed in the reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. A catalyst is a substance which speeds up a reaction, but is chemically. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID1803655 Why Do Catalysts Not Get Used Up Catalysts participate in a chemical reaction and increase its rate. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. When the reaction has finished, you would have. We can prove this by weighing our catalyst at the beginning. A catalyst is a substance which speeds up a reaction, but. Why Do Catalysts Not Get Used Up.
From www.youtube.com
Identifying catalysts in a reaction YouTube Why Do Catalysts Not Get Used Up They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts allow a reaction to. This page explains how adding a catalyst affects the rate of a reaction. Catalysts in biological reactions are called. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Why Do Catalysts Not Get Used Up This page explains how adding a catalyst affects the rate of a reaction. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. When a catalyst is added, it is not used up or chemically changed in the reaction. Catalysts participate in a chemical reaction and increase its rate. When the. Why Do Catalysts Not Get Used Up.
From chembam.com
Catalysts Why Do Catalysts Not Get Used Up Catalysts are substances that speed up a reaction but which are not. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts allow a reaction to. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. Why Do Catalysts Not Get Used Up.
From slideplayer.com
Catalysts Connect Starter Name as many compounds as you can ppt Why Do Catalysts Not Get Used Up They do not appear in the reaction’s net equation and are not consumed during the reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts are substances that speed up a reaction but which are not. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at. Why Do Catalysts Not Get Used Up.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Why Do Catalysts Not Get Used Up When a catalyst is added, it is not used up or chemically changed in the reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. The greater the frequency of successful collisions between. Why Do Catalysts Not Get Used Up.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Why Do Catalysts Not Get Used Up We can prove this by weighing our catalyst at the beginning. Catalysts are substances that speed up a reaction but which are not. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is a. Why Do Catalysts Not Get Used Up.
From www.slideshare.net
Biology 2.4 Why Do Catalysts Not Get Used Up If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in. Why Do Catalysts Not Get Used Up.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Why Do Catalysts Not Get Used Up Catalysts are substances that speed up a reaction but which are not. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page explains how adding a catalyst affects the rate of a reaction. If. Why Do Catalysts Not Get Used Up.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. When the reaction has finished, you would have. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. When a catalyst is added, it is not used up or chemically changed in the reaction. This page explains. Why Do Catalysts Not Get Used Up.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts are substances that speed up a reaction but which are not. They do not appear in the reaction’s net equation and are not consumed during. Why Do Catalysts Not Get Used Up.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts allow a reaction to. When the reaction has finished, you would have. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. Why Do Catalysts Not Get Used Up.
From www.slideshare.net
Catalyst Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts participate in a chemical reaction and increase its rate. This page explains how adding a catalyst affects the rate of a reaction. We can prove this by weighing our catalyst at the beginning. Catalysts have no effect on the equilibrium. Why Do Catalysts Not Get Used Up.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Why Do Catalysts Not Get Used Up The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. When a catalyst is added, it is not used up or chemically changed in the reaction. Catalysts are substances that speed up a reaction but which are not.. Why Do Catalysts Not Get Used Up.
From sop4cv.com
Catalysis Why Do Catalysts Not Get Used Up When a catalyst is added, it is not used up or chemically changed in the reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. When the reaction has. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4784681 Why Do Catalysts Not Get Used Up They do not appear in the reaction’s net equation and are not consumed during the reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. We can prove this by weighing our catalyst at the beginning. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. Why Do Catalysts Not Get Used Up.
From brainly.com
Which of the following statements about catalysts is false? A catalyst Why Do Catalysts Not Get Used Up When a catalyst is added, it is not used up or chemically changed in the reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. When the reaction has finished, you would have. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Why Do Catalysts Not Get Used Up Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. Why Do Catalysts Not Get Used Up.
From fyoniugms.blob.core.windows.net
Why Does Catalyst Do Not Need To Be Replaced Frequently at Deborah Hoy blog Why Do Catalysts Not Get Used Up A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts are substances that speed up a reaction but which are not. Catalysts participate in a chemical reaction and increase its rate. When a catalyst is added, it is not used up or chemically changed in the reaction. Catalysts in. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Why Do Catalysts Not Get Used Up Catalysts are substances that speed up a reaction but which are not. When the reaction has finished, you would have. They do not appear in the reaction’s net equation and are not consumed during the reaction. We can prove this by weighing our catalyst at the beginning. Catalysts allow a reaction to. Catalysts in biological reactions are called enzymes close. Why Do Catalysts Not Get Used Up.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Why Do Catalysts Not Get Used Up They do not appear in the reaction’s net equation and are not consumed during the reaction. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or. Why Do Catalysts Not Get Used Up.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Why Do Catalysts Not Get Used Up This page explains how adding a catalyst affects the rate of a reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts are substances that speed up a reaction. Why Do Catalysts Not Get Used Up.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect Why Do Catalysts Not Get Used Up The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page explains how adding a catalyst affects the rate of a reaction. Catalysts allow a reaction to. When a catalyst is added, it is not used up or chemically changed in the reaction. Catalysts participate in a chemical reaction and increase its rate. When. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4784681 Why Do Catalysts Not Get Used Up This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts allow a reaction to. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end. Why Do Catalysts Not Get Used Up.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Why Do Catalysts Not Get Used Up A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. When a catalyst is added, it is not used up or chemically changed in the reaction. If a catalyst is consumed in the reaction. Why Do Catalysts Not Get Used Up.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Why Do Catalysts Not Get Used Up We can prove this by weighing our catalyst at the beginning. When the reaction has finished, you would have. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts have no effect on the equilibrium constant and thus on. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Why Do Catalysts Not Get Used Up It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts are substances that speed up a reaction but which are not. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Why Do Catalysts Not Get Used Up This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. We can prove this by weighing our catalyst at the beginning. When the reaction has finished, you would have. When a catalyst is added, it is not. Why Do Catalysts Not Get Used Up.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Why Do Catalysts Not Get Used Up Catalysts allow a reaction to. We can prove this by weighing our catalyst at the beginning. Catalysts participate in a chemical reaction and increase its rate. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. If a catalyst is consumed in. Why Do Catalysts Not Get Used Up.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Why Do Catalysts Not Get Used Up They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. When the reaction has finished, you would have. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. When a catalyst is added, it is. Why Do Catalysts Not Get Used Up.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Why Do Catalysts Not Get Used Up We can prove this by weighing our catalyst at the beginning. Catalysts participate in a chemical reaction and increase its rate. Catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts allow a reaction to.. Why Do Catalysts Not Get Used Up.
From www.pinterest.com
The catalyst can change the speed of a reaction without being used up Why Do Catalysts Not Get Used Up Catalysts allow a reaction to. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. This page explains how adding a catalyst affects the rate of a reaction. Catalysts participate in a chemical reaction and. Why Do Catalysts Not Get Used Up.