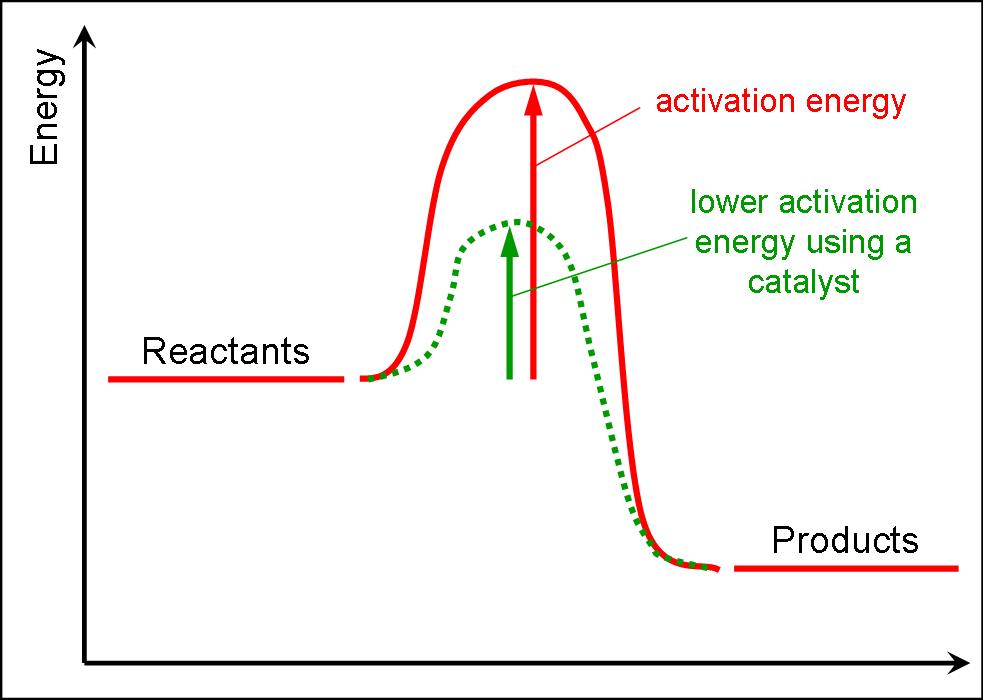
Activation Energy Change Catalyst . learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing ea and increasing the reaction rate. One possible way of doing this. catalysts and activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. To increase the rate of a reaction you need to increase the number of successful collisions. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The catalyst participates in intermediate steps of the. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. See some examples of catalysts.
from exyepzauq.blob.core.windows.net
Catalysts are defined as substances that participate in a chemical reaction but. See some examples of catalysts. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. catalysts provide a means of reducing ea and increasing the reaction rate. One possible way of doing this. catalysts and activation energy. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. To increase the rate of a reaction you need to increase the number of successful collisions. learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts.
Enzymes Help To Lower The Activation Energies Of Reaction By at Laura
Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts provide a means of reducing ea and increasing the reaction rate. The catalyst participates in intermediate steps of the. See some examples of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this. learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts.
From socratic.org
What are activation energies? Socratic Activation Energy Change Catalyst One possible way of doing this. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts and activation energy. Catalysts are defined as substances that participate in a chemical reaction but. in. Activation Energy Change Catalyst.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Activation Energy Change Catalyst catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. catalysts provide a means of reducing ea and increasing the reaction rate. See some examples of catalysts.. Activation Energy Change Catalyst.
From animalia-life.club
Enzyme Activation Energy Graph Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. catalysts provide a means of reducing ea and increasing the reaction rate. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Catalysts are defined as substances that participate in a chemical reaction but. See. Activation Energy Change Catalyst.
From www.animalia-life.club
Enzyme Graph Transition State Activation Energy Change Catalyst learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. See some examples of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing ea and increasing the reaction rate. in chemistry and physics, activation energy is the. Activation Energy Change Catalyst.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts are defined as substances that participate in a chemical reaction but. The catalyst participates in intermediate steps of the. catalysts and. Activation Energy Change Catalyst.
From studylib.net
Catalysts and Activation Energy 2H2O2 (aq) → 2H2O (l) + O2 (g Activation Energy Change Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts provide a means of reducing ea and increasing. Activation Energy Change Catalyst.
From www.expii.com
Energy Diagram — Overview & Parts Expii Activation Energy Change Catalyst in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The catalyst participates in intermediate steps of the. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts are defined as substances that participate in a chemical reaction but. See some examples of catalysts.. Activation Energy Change Catalyst.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Activation Energy Change Catalyst learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts provide a means. Activation Energy Change Catalyst.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Activation Energy Change Catalyst One possible way of doing this. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. a catalyst changes the mechanism of a chemical reaction and lowers its activation. Activation Energy Change Catalyst.
From www.slideserve.com
PPT KEY CONCEPT Enzymes are catalysts for chemical reactions in Activation Energy Change Catalyst in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. One possible way of doing this. catalysts and activation energy. See some examples of catalysts. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. The catalyst participates in intermediate steps of the. in. Activation Energy Change Catalyst.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. One possible way of doing this. The catalyst participates in. Activation Energy Change Catalyst.
From exyfvrfps.blob.core.windows.net
Do All Catalysts Lower Activation Energy at Shirley Tolliver blog Activation Energy Change Catalyst Catalysts are defined as substances that participate in a chemical reaction but. The catalyst participates in intermediate steps of the. One possible way of doing this. learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. catalysts provide a means of reducing ea and increasing the reaction rate.. Activation Energy Change Catalyst.
From www.youtube.com
Enthalpy Change, Activation Energy, Catalysts & Temperature // Prelim Activation Energy Change Catalyst learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. One possible way of doing this. See some examples of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. To increase the rate of a reaction you need to increase the number of successful collisions.. Activation Energy Change Catalyst.
From www.slideserve.com
PPT An Introduction to Metabolism PowerPoint Presentation, free Activation Energy Change Catalyst The catalyst participates in intermediate steps of the. To increase the rate of a reaction you need to increase the number of successful collisions. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts provide a means of reducing ea and increasing the reaction rate. a catalyst changes. Activation Energy Change Catalyst.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Activation Energy Change Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts are defined as substances that participate in a chemical reaction but. catalysts and activation energy. One possible way of doing this. The catalyst participates in intermediate steps of the. learn what the activation energy of a chemical reaction is and how. Activation Energy Change Catalyst.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Activation Energy Change Catalyst catalysts and activation energy. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. See some examples of catalysts. The catalyst participates in intermediate steps of the. catalysts provide a means of reducing ea and increasing the reaction rate. To increase the rate of a reaction you need to increase the number of. Activation Energy Change Catalyst.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Activation Energy Change Catalyst catalysts provide a means of reducing ea and increasing the reaction rate. See some examples of catalysts. One possible way of doing this. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts and activation energy. a catalyst changes the mechanism of a chemical reaction and lowers. Activation Energy Change Catalyst.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Activation Energy Change Catalyst One possible way of doing this. catalysts and activation energy. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. See some examples of catalysts. in chemistry and physics, activation energy is the minimum amount of energy. Activation Energy Change Catalyst.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Activation Energy Change Catalyst One possible way of doing this. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing ea and increasing the reaction rate. in the case of a biological reaction, when an enzyme (a form of. Activation Energy Change Catalyst.
From iconpasob.blogg.se
iconpasob.blogg.se How much does catalyst need of activation energy Activation Energy Change Catalyst in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The catalyst participates in intermediate steps of the. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. See some examples of catalysts. learn what the activation. Activation Energy Change Catalyst.
From 2012books.lardbucket.org
Catalysis Activation Energy Change Catalyst in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts and activation energy. The catalyst participates in intermediate steps of the. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. learn what the activation energy of a chemical reaction is and how. Activation Energy Change Catalyst.
From exyvshuta.blob.core.windows.net
Catalyst In Chemistry Example at Sharon Kane blog Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. learn what the activation energy. Activation Energy Change Catalyst.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. catalysts and activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. catalysts provide a means of reducing ea and increasing the reaction rate. Catalysts are defined as. Activation Energy Change Catalyst.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. One possible way of doing this. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Catalysts are defined as substances that participate in a chemical reaction but.. Activation Energy Change Catalyst.
From www.pinterest.ch
Enzymes are biological catalysts that change the rate of the reaction Activation Energy Change Catalyst The catalyst participates in intermediate steps of the. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this. catalysts provide a means of reducing ea and increasing the. Activation Energy Change Catalyst.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Activation Energy Change Catalyst learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. To increase the rate of a reaction you need to increase the number of successful collisions. catalysts and activation energy. One possible way of doing this. See some examples of catalysts. in chemistry and physics, activation energy. Activation Energy Change Catalyst.
From fyoulxtwt.blob.core.windows.net
How Do Catalysts Work Gcse at Sharon Santucci blog Activation Energy Change Catalyst learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. See some examples of catalysts. catalysts provide a means of reducing ea and increasing the reaction rate. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. a. Activation Energy Change Catalyst.
From exyepzauq.blob.core.windows.net
Enzymes Help To Lower The Activation Energies Of Reaction By at Laura Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts provide a means of reducing ea and increasing the reaction rate. One possible way of doing this. in the case of a biological. Activation Energy Change Catalyst.
From ar.inspiredpencil.com
Catalyst Activation Energy Change Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. See some examples of catalysts. catalysts and activation energy. One possible way of doing this. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The catalyst participates in intermediate steps of the. . Activation Energy Change Catalyst.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Activation Energy Change Catalyst catalysts and activation energy. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. Catalysts are defined as substances that participate in a chemical reaction but. See some examples of catalysts. The catalyst participates in intermediate steps of the. in the case of a biological reaction, when an enzyme (a form of catalyst). Activation Energy Change Catalyst.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Activation Energy Change Catalyst a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. catalysts provide a means of reducing ea and increasing the reaction rate. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this. learn what the activation. Activation Energy Change Catalyst.
From www.vedantu.com
A catalyst increases the rate of reaction by……A. Decreasing enthalpyB Activation Energy Change Catalyst catalysts and activation energy. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. catalysts provide a means of reducing ea and increasing the reaction rate. a catalyst changes the mechanism of a chemical reaction and lowers its activation energy. learn what the activation energy of a. Activation Energy Change Catalyst.
From brainly.com
Classify each statement about catalysts as true or false. Catalyst Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. The catalyst participates in intermediate steps of the. See some examples of catalysts. learn what the activation energy of a chemical reaction is and how it is lowered with the use of catalysts. a catalyst. Activation Energy Change Catalyst.
From www.reddit.com
Why does enthalpy of reaction with temperature change by Kirchoff's Law Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. catalysts provide a means of reducing ea and increasing the reaction rate. See some examples of catalysts. Catalysts are defined as substances that participate in a chemical reaction but. a catalyst changes the mechanism of. Activation Energy Change Catalyst.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Activation Energy Change Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. To increase the rate of a reaction you need to increase the number of successful collisions. in chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. See some. Activation Energy Change Catalyst.