Dilution And Its Importance . dilution is the addition of solvent, which decreases the concentration of the solute in the solution. why dilution is important? Dilution of solutions helps us in many ways. Concentration is the removal of solvent, which. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. During titration, solutions are diluted so there is a larger volume of solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by.
from www.labpedia.net
solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. learn how to dilute and concentrate solutions. Dilution of solutions helps us in many ways. During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
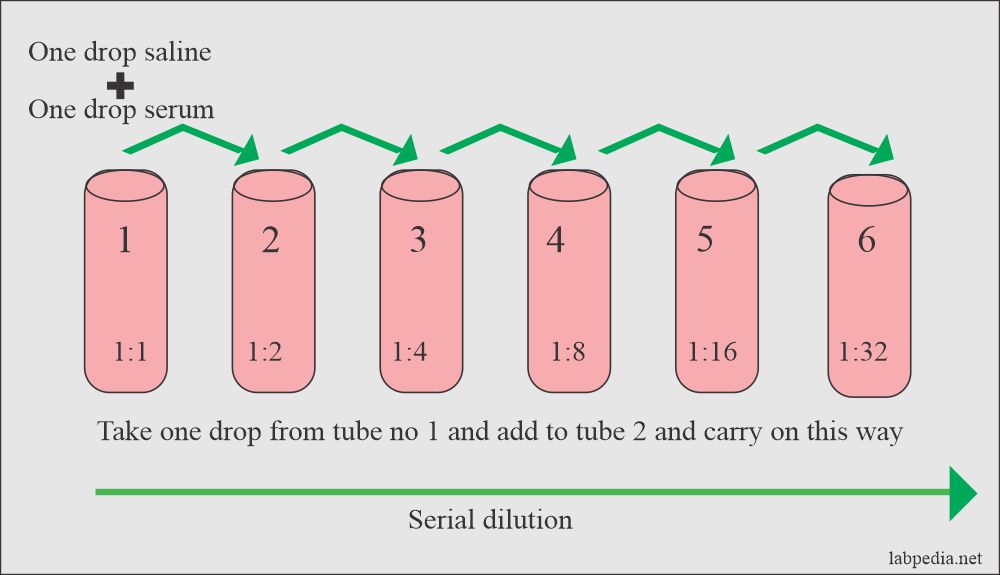
Enteric fever Part 2 Typhoid / Enteric fever Diagnosis, Widal test
Dilution And Its Importance Concentration is the removal of solvent, which. learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. why dilution is important? solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. Dilution of solutions helps us in many ways. During titration, solutions are diluted so there is a larger volume of solution. Often, a worker will need to change the concentration of a solution by.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution And Its Importance solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution of solutions helps us in many ways. dilution is the addition of solvent, which decreases the concentration of the solute. Dilution And Its Importance.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution And Its Importance learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there. Dilution And Its Importance.
From giohpvbek.blob.core.windows.net
Dilution Crypto Meaning at Dorothy Bell blog Dilution And Its Importance why dilution is important? solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. . Dilution And Its Importance.
From serreweightloss.weebly.com
The importance of serial dilution serreweightloss Dilution And Its Importance learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solution concentrations are typically. Dilution And Its Importance.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution And Its Importance During titration, solutions are diluted so there is a larger volume of solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. why dilution is important? Often,. Dilution And Its Importance.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution And Its Importance a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. why dilution is important? dilution is the addition of solvent, which decreases the concentration of the solute. Dilution And Its Importance.
From serreweightloss.weebly.com
The importance of serial dilution serreweightloss Dilution And Its Importance Often, a worker will need to change the concentration of a solution by. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions. why dilution is important? Concentration is the removal of solvent, which. dilution refers to the process of. Dilution And Its Importance.
From letstalkscience.ca
Osmosis and Its Role in Human Biology and Health Let's Talk Science Dilution And Its Importance a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution of solutions helps us in many ways. learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution. dilution refers to the process of preparing a solution with. Dilution And Its Importance.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution And Its Importance Concentration is the removal of solvent, which. why dilution is important? During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution And Its Importance.
From ar.inspiredpencil.com
Serial Dilution Diagram Dilution And Its Importance solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. why dilution is important? During titration, solutions are diluted so there is a larger volume of solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution. Dilution And Its Importance.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution And Its Importance learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there is a relatively small amount of. Dilution And Its Importance.
From fyoezjbli.blob.core.windows.net
Dilution Technique For Blood Volume at Anne Cox blog Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of. Dilution And Its Importance.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. Often, a worker will need to change the concentration of a solution by. Dilution of solutions helps us in. Dilution And Its Importance.
From www.labpedia.net
Enteric fever Part 2 Typhoid / Enteric fever Diagnosis, Widal test Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. Dilution of solutions helps us in many ways. learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration. Dilution And Its Importance.
From exycznfma.blob.core.windows.net
Tube Dilution And Agar Plate Method at Paul Everhart blog Dilution And Its Importance learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. Dilution of solutions helps us in many ways. dilution is the. Dilution And Its Importance.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. Dilution of solutions helps us in many ways. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.. Dilution And Its Importance.
From fyolgejsd.blob.core.windows.net
Why Serial Dilution Is Important In Microbiology at Margaret Allen blog Dilution And Its Importance a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. dilution refers to the process of preparing a solution with a. Dilution And Its Importance.
From fyolgejsd.blob.core.windows.net
Why Serial Dilution Is Important In Microbiology at Margaret Allen blog Dilution And Its Importance During titration, solutions are diluted so there is a larger volume of solution. Concentration is the removal of solvent, which. why dilution is important? dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of. Dilution And Its Importance.
From retpaunder.weebly.com
Importance Of Serial Dilution In Serological Tests retpaunder Dilution And Its Importance dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. Dilution of solutions helps us in many ways. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases. Dilution And Its Importance.
From www.differencebetween.com
Difference Between Dilution and Concentration Compare the Difference Dilution And Its Importance why dilution is important? solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution of solutions helps us in many ways. a dilute solution is one in which there. Dilution And Its Importance.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilution And Its Importance dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. learn how to dilute and concentrate solutions. why dilution is important? Often, a worker will need to change the concentration of a solution by. a dilute solution is one in which there is a relatively. Dilution And Its Importance.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution And Its Importance.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution And Its Importance Often, a worker will need to change the concentration of a solution by. learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. During titration, solutions are diluted so there is a larger volume of solution. solution concentrations are typically expressed as molarities and. Dilution And Its Importance.
From www.coursehero.com
[Solved] 1. For each dilution, count the number of colony... Course Hero Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. why dilution is important? learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. Concentration is the removal of solvent, which. Often,. Dilution And Its Importance.
From www.integra-biosciences.com
Guide Learn how to perform serial dilutions with INTEGRA INTEGRA Dilution And Its Importance why dilution is important? Often, a worker will need to change the concentration of a solution by. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in. Dilution And Its Importance.
From facts.net
13 Surprising Facts About Dilution Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. Concentration is the removal of solvent, which. why dilution is important? During titration, solutions are diluted so there is a. Dilution And Its Importance.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution And Its Importance solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. During titration, solutions are diluted so there is a larger volume of solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. Dilution of solutions helps. Dilution And Its Importance.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID9598576 Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. why dilution is important? learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution.. Dilution And Its Importance.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. a dilute solution is one in which there is a relatively small. Dilution And Its Importance.
From exyrfdlro.blob.core.windows.net
Dilution And Rate at Herb White blog Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent. Dilution And Its Importance.
From www.youtube.com
Serial Dilution Calculations Examples YouTube Dilution And Its Importance a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution. Dilution And Its Importance.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution And Its Importance a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. During titration, solutions are diluted so there is a larger volume of solution. learn how to dilute and concentrate solutions. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to. Dilution And Its Importance.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution And Its Importance learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent. Dilution And Its Importance.
From testbook.com
Dilution Learn its Definition, Formula, Method & Importance Dilution And Its Importance dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a. why dilution is important? dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution And Its Importance.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use Dilution And Its Importance dilution refers to the process of preparing a solution with a smaller concentration by adding a solvent to the original solution. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. why dilution is important? Concentration is the. Dilution And Its Importance.