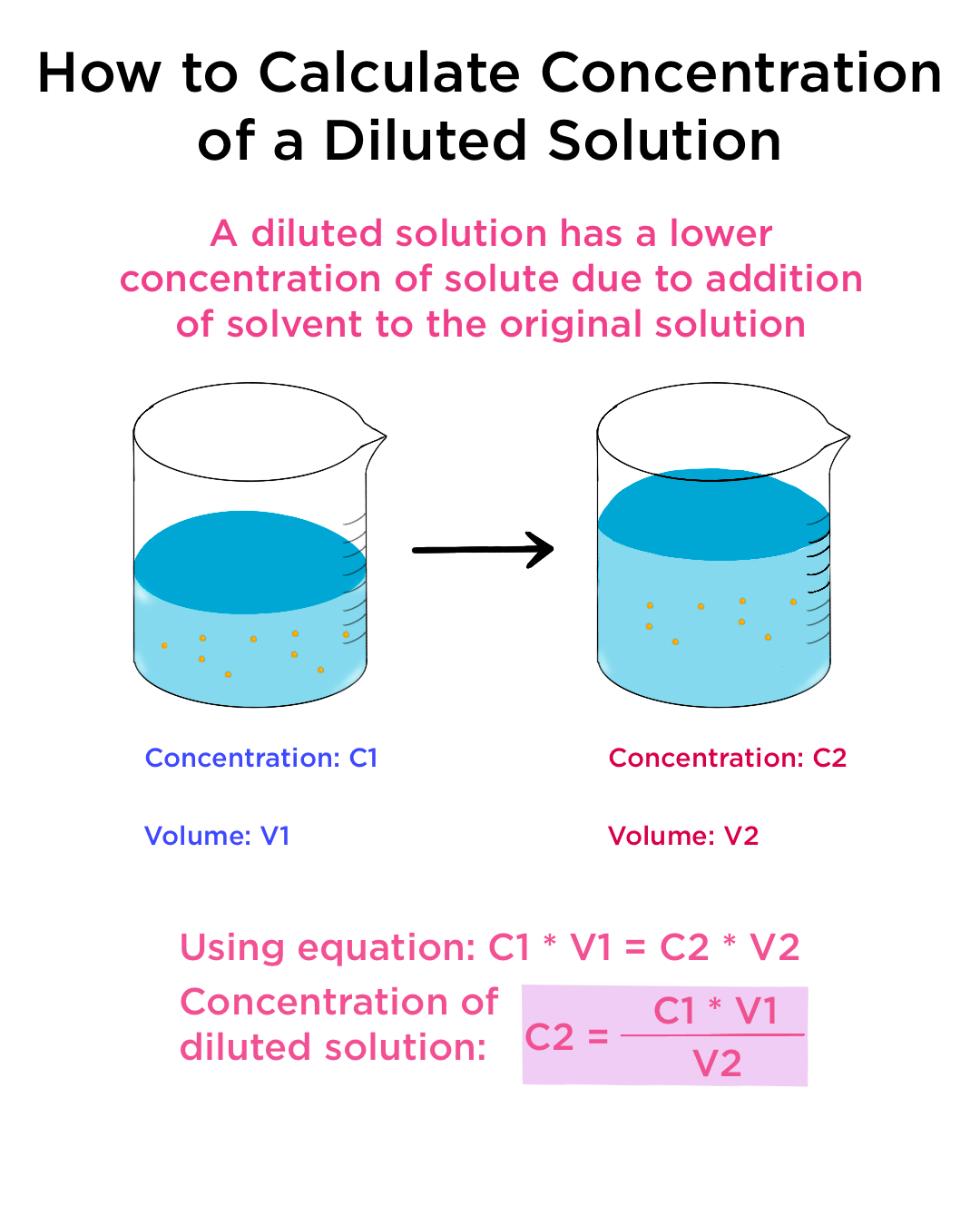
How To Work Out Concentration Of A Dilute Solution . M i v i = m f v f where m is molarity, v is. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. often, a worker will need to change the concentration of a solution by changing the amount of solvent. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. Dilution is the addition of solvent, which decreases the. you can calculate the concentration of a solution following a dilution by applying this equation: a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.
from ar.inspiredpencil.com
to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. Dilution is the addition of solvent, which decreases the. you can calculate the concentration of a solution following a dilution by applying this equation: to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. often, a worker will need to change the concentration of a solution by changing the amount of solvent. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. M i v i = m f v f where m is molarity, v is. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.
Concentrated Vs Dilute Solutions
How To Work Out Concentration Of A Dilute Solution to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. often, a worker will need to change the concentration of a solution by changing the amount of solvent. you can calculate the concentration of a solution following a dilution by applying this equation: therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. M i v i = m f v f where m is molarity, v is. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of solvent, which decreases the. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. M i v i = m f v f where m is molarity, v is. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. the answer is basically correct (see. How To Work Out Concentration Of A Dilute Solution.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint How To Work Out Concentration Of A Dilute Solution therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution How To Work Out Concentration Of A Dilute Solution to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. M i v i = m f v f where m is molarity, v is. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. a dilute solution is. How To Work Out Concentration Of A Dilute Solution.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 How To Work Out Concentration Of A Dilute Solution Dilution is the addition of solvent, which decreases the. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. a dilute solution is one in which there. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
[Example] How to Find the Concentration of a Solution. YouTube How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Calculate Concentration (Example) YouTube How To Work Out Concentration Of A Dilute Solution Dilution is the addition of solvent, which decreases the. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. M i v i = m f v f where. How To Work Out Concentration Of A Dilute Solution.
From www.slideserve.com
PPT Solution Concentration PowerPoint Presentation, free download How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
A chemist must dilute 75.0mL ml of 6.00M aqueous sodium nitrate How To Work Out Concentration Of A Dilute Solution M i v i = m f v f where m is molarity, v is. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. you can calculate the concentration of a solution following a dilution by applying this equation: therefore, in order to reliably control. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How To Work Out Concentration Of A Dilute Solution Dilution is the addition of solvent, which decreases the. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. often, a worker will need to change the concentration of a solution by changing the amount of solvent. to calculate the concentration of a diluted solution,. How To Work Out Concentration Of A Dilute Solution.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz How To Work Out Concentration Of A Dilute Solution often, a worker will need to change the concentration of a solution by changing the amount of solvent. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. the answer is basically correct (see note at the end of my answer about significant figures) but there is a. How To Work Out Concentration Of A Dilute Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution Dilution is the addition of solvent, which decreases the. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. you can calculate the concentration of a solution following a dilution by applying this equation: to calculate the concentration of a diluted solution, you use the formula. How To Work Out Concentration Of A Dilute Solution.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science How To Work Out Concentration Of A Dilute Solution Dilution is the addition of solvent, which decreases the. you can calculate the concentration of a solution following a dilution by applying this equation: often, a worker will need to change the concentration of a solution by changing the amount of solvent. therefore, in order to reliably control the process of changing the concentration of a homogeneous. How To Work Out Concentration Of A Dilute Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts How To Work Out Concentration Of A Dilute Solution a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent, which decreases the. therefore, in order to reliably control the process of changing the. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. often, a worker will need to change the concentration of a solution by changing the amount of solvent. M i v i = m f v f where m is molarity, v is. the answer is. How To Work Out Concentration Of A Dilute Solution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of solvent, which decreases the. often, a worker will need to change the. How To Work Out Concentration Of A Dilute Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. M i v i = m f v f where m is molarity, v is. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. a dilute solution. How To Work Out Concentration Of A Dilute Solution.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. M i v i = m f v f where m is molarity, v is. often, a worker will. How To Work Out Concentration Of A Dilute Solution.
From letstalkscience.ca
Osmosis and Its Role in Human Biology and Health Let's Talk Science How To Work Out Concentration Of A Dilute Solution therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of solvent, which decreases the. the answer is basically correct (see note at the end. How To Work Out Concentration Of A Dilute Solution.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID3874312 How To Work Out Concentration Of A Dilute Solution M i v i = m f v f where m is molarity, v is. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilute solution is one in which. How To Work Out Concentration Of A Dilute Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution you can calculate the concentration of a solution following a dilution by applying this equation: to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler. How To Work Out Concentration Of A Dilute Solution.
From quizlet.com
Mixtures and Solutions Diagram Quizlet How To Work Out Concentration Of A Dilute Solution M i v i = m f v f where m is molarity, v is. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. you. How To Work Out Concentration Of A Dilute Solution.
From brainly.in
how can a solution be dilute or concentrated Brainly.in How To Work Out Concentration Of A Dilute Solution often, a worker will need to change the concentration of a solution by changing the amount of solvent. M i v i = m f v f where m is molarity, v is. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. Dilution is the addition. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Diluting a 12 M stock solution YouTube How To Work Out Concentration Of A Dilute Solution you can calculate the concentration of a solution following a dilution by applying this equation: Dilution is the addition of solvent, which decreases the. M i v i = m f v f where m is molarity, v is. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. therefore,. How To Work Out Concentration Of A Dilute Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. M i v i = m f v f where m is molarity, v is. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,.. How To Work Out Concentration Of A Dilute Solution.
From socratic.org
mL of solution has a concentration of 3.0 M. How much water needs to be How To Work Out Concentration Of A Dilute Solution the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. often, a worker will need to change the concentration of a solution by changing the amount of solvent. you can calculate the concentration of a solution following a dilution by applying this equation: M i. How To Work Out Concentration Of A Dilute Solution.
From slideplayer.com
Solution Concentration ppt download How To Work Out Concentration Of A Dilute Solution a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. often, a worker will need to change the concentration of a solution by changing the amount of solvent. you can calculate the concentration of a solution following a dilution by applying this equation: to calculate the concentration. How To Work Out Concentration Of A Dilute Solution.
From saylordotorg.github.io
Solution Concentrations How To Work Out Concentration Of A Dilute Solution to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. you can calculate the concentration of a solution following a dilution by applying this equation: to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. the answer is. How To Work Out Concentration Of A Dilute Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions How To Work Out Concentration Of A Dilute Solution you can calculate the concentration of a solution following a dilution by applying this equation: to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. therefore,. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
1.3 Solve Problems Using Concentration, Amount of Solute and Volume [SL How To Work Out Concentration Of A Dilute Solution to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. the answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method,. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount. How To Work Out Concentration Of A Dilute Solution.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Work Out Concentration Of A Dilute Solution M i v i = m f v f where m is molarity, v is. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. a dilute. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration How To Work Out Concentration Of A Dilute Solution to make a dilution, you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. M i v i = m f v f where m is molarity, v is. you can calculate the concentration of a solution following a dilution by applying this equation: Dilution is the addition of solvent, which. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Dilution determining final concentration (example) YouTube How To Work Out Concentration Of A Dilute Solution to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. M i v i = m f v f where m is molarity, v is. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. often, a worker will need to. How To Work Out Concentration Of A Dilute Solution.
From gionuxzhl.blob.core.windows.net
Dilute A Test at Cedric Santiago blog How To Work Out Concentration Of A Dilute Solution you can calculate the concentration of a solution following a dilution by applying this equation: therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. How To Work Out Concentration Of A Dilute Solution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How To Work Out Concentration Of A Dilute Solution you can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is. therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. to make a dilution, you simply add a. How To Work Out Concentration Of A Dilute Solution.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture How To Work Out Concentration Of A Dilute Solution therefore, in order to reliably control the process of changing the concentration of a homogeneous mixture, more solvent must. Dilution is the addition of solvent, which decreases the. to calculate the concentration of a diluted solution, you use the formula c1v 1 = c2v 2. often, a worker will need to change the concentration of a solution. How To Work Out Concentration Of A Dilute Solution.