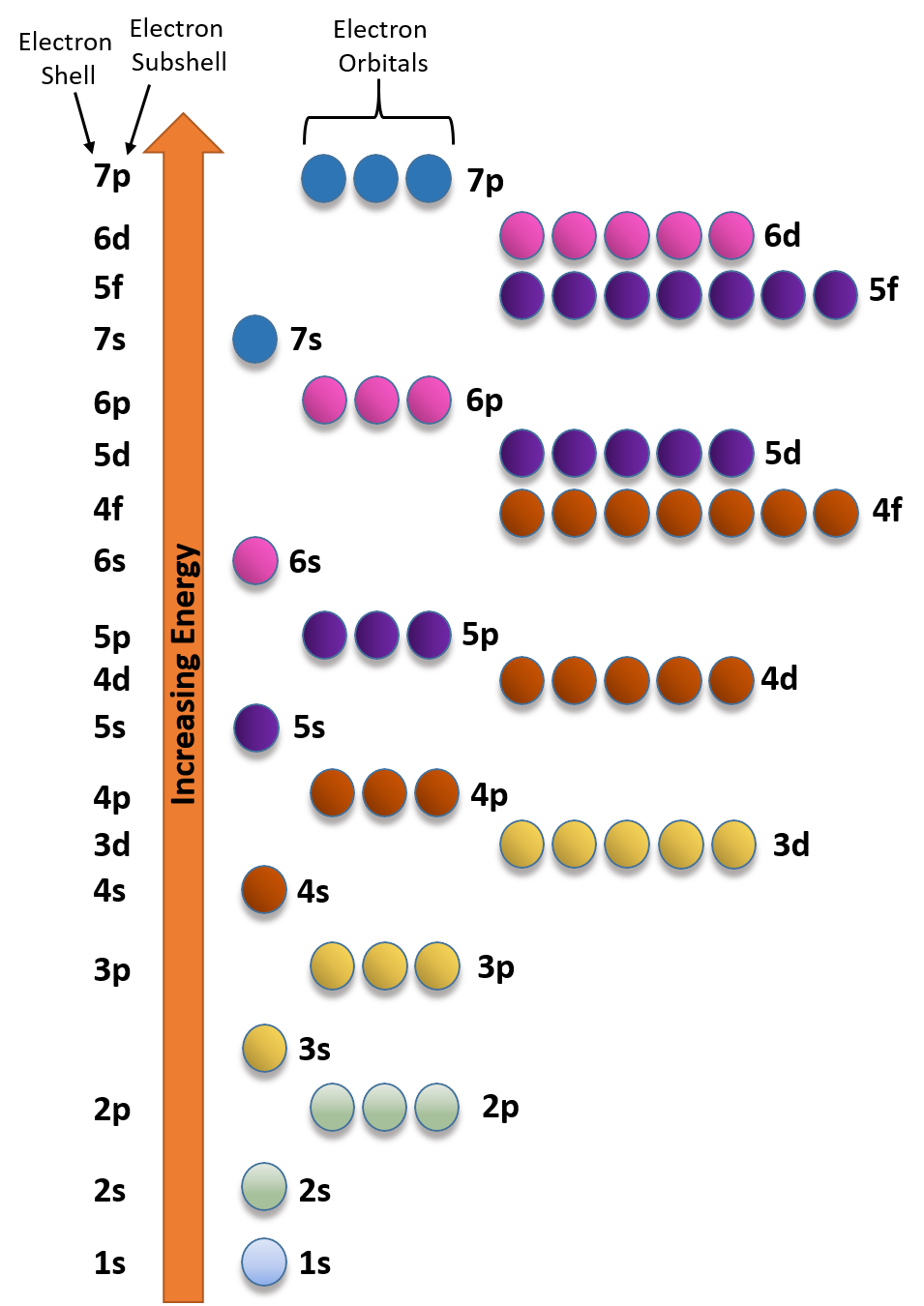
Iron Three Electrons . The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. The purple potassium ferrate (k 2. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. They orbit the nucleus in. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron also occurs in higher oxidation states, e.g. By the end of this section, you will be able to: Fe, fe2+, and fe3+ electron. Iron has 26 electrons, matching the number of protons.
from wou.edu
When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Fe, fe2+, and fe3+ electron. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron also occurs in higher oxidation states, e.g. The purple potassium ferrate (k 2. Iron has 26 electrons, matching the number of protons. Electrons are negatively charged particles.
CH150 Chapter 2 Atoms and Periodic Table Chemistry
Iron Three Electrons Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron also occurs in higher oxidation states, e.g. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Fe, fe2+, and fe3+ electron. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. They orbit the nucleus in. The purple potassium ferrate (k 2. By the end of this section, you will be able to: When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). Iron has 26 electrons, matching the number of protons. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Three Electrons The purple potassium ferrate (k 2. By the end of this section, you will be able to: Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Electrons are negatively charged particles. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The electronic configuration of. Iron Three Electrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Three Electrons Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Fe, fe2+, and fe3+ electron. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Electrons are negatively charged particles. The purple potassium ferrate (k 2. Iron. Iron Three Electrons.
From new--electronic.blogspot.com
Electron Configuration Of Ferrous Ion Iron Three Electrons Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). Electrons are negatively charged particles. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. By the end of this section, you will be able to: Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#,. Iron Three Electrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Three Electrons Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). By the end of this section, you will be able to: When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron also occurs in higher oxidation states, e.g. The protons and neutrons are contained in. Iron Three Electrons.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Three Electrons Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. By the end of this section, you will be able to: Electrons are negatively charged particles. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Iron has 26 electrons, matching the number of protons. Iron also occurs in higher oxidation. Iron Three Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Iron Three Electrons By the end of this section, you will be able to: Electrons are negatively charged particles. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron has 26 electrons, matching the number of protons. Fe, fe2+, and fe3+ electron. Iron also occurs in. Iron Three Electrons.
From slideplayer.com
Chapter 4 Electron Arrangement. ppt download Iron Three Electrons Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Iron has 26 electrons, matching the number of protons. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. They orbit the nucleus in. The electronic configuration of fe2+ is 1s2 2s2. Iron Three Electrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron has 26 electrons, matching the number of protons. By the end of this section, you will be able to:. Iron Three Electrons.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Fe, fe2+, and fe3+ electron. The purple potassium ferrate (k 2. Iron also occurs in higher oxidation states, e.g. Iron forms compounds. Iron Three Electrons.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Iron also occurs in higher oxidation states, e.g. By the end of this. Iron Three Electrons.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Iron Three Electrons Iron has 26 electrons, matching the number of protons. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron also occurs in higher oxidation states, e.g. Fe, fe2+, and fe3+ electron. The electronic configuration of fe2+ is 1s2 2s2. Iron Three Electrons.
From www.researchgate.net
Configurations of the 3d electrons of Fe(II) and Fe(III), their Iron Three Electrons Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Iron also occurs in higher oxidation states, e.g. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). Atoms are in fact made up of three smaller. Iron Three Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Three Electrons Iron also occurs in higher oxidation states, e.g. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The purple potassium ferrate (k. Iron Three Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Three Electrons Iron has 26 electrons, matching the number of protons. Iron also occurs in higher oxidation states, e.g. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. They orbit the nucleus in. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The protons and neutrons are contained. Iron Three Electrons.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Three Electrons Iron has 26 electrons, matching the number of protons. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Iron also occurs in higher oxidation states, e.g. Fe, fe2+, and fe3+ electron. Iron exhibits these three allotropic forms at different temperatures when it. Iron Three Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Three Electrons Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. They orbit the nucleus in. Iron has 26 electrons, matching the number of protons.. Iron Three Electrons.
From www.numerade.com
SOLVED TRE For the reaction 16Fe(s) + 3Ss (8) + 8Fe2S3(s) choose how Iron Three Electrons By the end of this section, you will be able to: The purple potassium ferrate (k 2. They orbit the nucleus in. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron also occurs in higher oxidation states, e.g. Iron has 26 electrons, matching the number of protons.. Iron Three Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Iron Three Electrons Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. The purple potassium ferrate (k 2. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). The protons and neutrons are contained in the nucleus, the tiny centre. Iron Three Electrons.
From galvinconanstuart.blogspot.com
Which Particle Diagram Represents A Mixture Of Three Substances Iron Three Electrons Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Iron also occurs in higher oxidation states, e.g. The purple potassium ferrate (k 2. Fe, fe2+, and fe3+ electron. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. They orbit the nucleus in. When we write the configuration we'll put. Iron Three Electrons.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Three Electrons Electrons are negatively charged particles. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The purple potassium ferrate (k 2. Iron has 26 electrons, matching the number of protons. Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). The protons and neutrons are contained in the nucleus, the tiny centre of an. Iron Three Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. By the end of this section, you will be able to: They orbit the nucleus in. Iron is on the fourth row of. Iron Three Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Iron Three Electrons Iron has 26 electrons, matching the number of protons. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. They orbit the nucleus in. Iron. Iron Three Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Three Electrons They orbit the nucleus in. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Electrons are negatively charged particles. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Iron also occurs in higher oxidation states,. Iron Three Electrons.
From www.numerade.com
SOLVEDA zinc (Zn) atom loses two electrons to an ion. An iron Iron Three Electrons Fe, fe2+, and fe3+ electron. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. When we write. Iron Three Electrons.
From chemguru.sg
High Spin and Low Spin Complexes Iron Three Electrons Iron has 26 electrons, matching the number of protons. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron forms compounds mainly in the oxidation states. Iron Three Electrons.
From worksheet-z.netlify.app
Electron Configuration Of Iron (iii) Ion Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. The purple potassium ferrate (k 2. When we write the. Iron Three Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Three Electrons The purple potassium ferrate (k 2. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Iron also occurs in higher oxidation states, e.g. They orbit the nucleus in. When we write the configuration. Iron Three Electrons.
From ar.inspiredpencil.com
Iron Atomic Structure Iron Three Electrons By the end of this section, you will be able to: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Iron also occurs in higher oxidation states, e.g. The protons and neutrons are contained. Iron Three Electrons.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo (Free Trial) Bigstock Iron Three Electrons Iron forms compounds mainly in the oxidation states +2 (iron(ii), “ferrous”) and +3 (iron(iii), “ferric”). They orbit the nucleus in. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. Iron also occurs in higher oxidation states, e.g. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of. Iron Three Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Iron Three Electrons By the end of this section, you will be able to: Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Iron has 26 electrons, matching the number of protons. Fe, fe2+, and fe3+ electron. Iron also occurs in higher oxidation. Iron Three Electrons.
From awesomehome.co
Iron Periodic Table Electrons Awesome Home Iron Three Electrons Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. Iron also occurs in higher oxidation states, e.g. The purple potassium ferrate (k 2. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#,. Iron Three Electrons.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Three Electrons By the end of this section, you will be able to: Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6. Iron Three Electrons.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry Iron Three Electrons Atoms are in fact made up of three smaller particles called protons, electrons and neutrons. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Electrons are negatively charged particles. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron is on the fourth row of the periodic table,. Iron Three Electrons.
From www.nuclear-power.com
Iron Electron Affinity Electronegativity Ionization Energy of Iron Three Electrons The purple potassium ferrate (k 2. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. By the end of this section, you will be able to: The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe, fe2+, and fe3+ electron. Iron also occurs in higher. Iron Three Electrons.
From www.chegg.com
Solved What is the electron configuration of the Iron(III) Iron Three Electrons The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller. Atoms are in fact made up of three smaller particles. Iron Three Electrons.