Non Prescription Labelling . This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are.
from karmelleven-platform.blogspot.com
Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling.
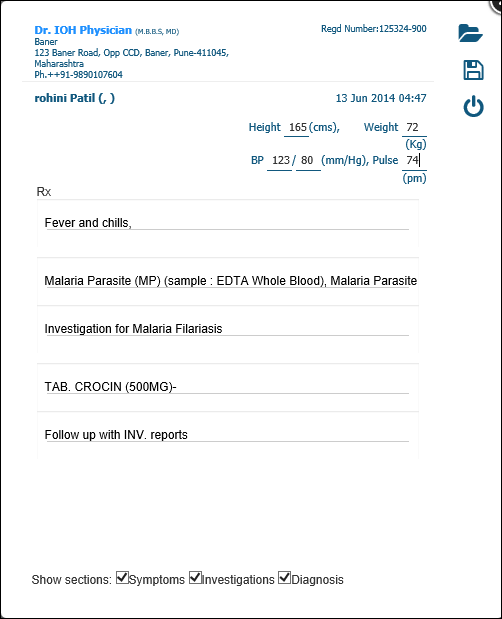
Labeled Prescription With Doctors Name Sample Labelling Requirements
Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling.
From delltech.com
Plain Language Labelling Regulations for NonPrescription Drugs Dell Tech Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to the hpra.. Non Prescription Labelling.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. This guidance explains the legal framework for. Non Prescription Labelling.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a. Non Prescription Labelling.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Non Prescription Labelling Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Non Prescription Labelling.
From fabalabse.com
What is label and its examples? Leia aqui What are 2 examples of Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to. Non Prescription Labelling.
From www.cutsheetlabels.com
Custom & Templated Pharmacy labels Cut Sheet Labels Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to. Non Prescription Labelling.
From www.unitedadlabel.com
How To Label Medications Properly United Ad Label Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Non Prescription Labelling.
From www.slideserve.com
PPT Labelling of Certain Paediatric NonPrescription Cough and Cold Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that. Non Prescription Labelling.
From www.canada.ca
Labelling requirements for nonprescription drugs guidance document Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and. Non Prescription Labelling.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements. Non Prescription Labelling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Non Prescription Labelling Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a. Non Prescription Labelling.
From www.umc.edu
Medication labels University of Mississippi Medical Center Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the. Non Prescription Labelling.
From www.slideserve.com
PPT Drug and Product Labeling PowerPoint Presentation, free download Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to the hpra.. Non Prescription Labelling.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure. Non Prescription Labelling.
From blog.caresfield.com
Medication Labels 101 Categories, Regulations, and Best Practices Non Prescription Labelling Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a. Non Prescription Labelling.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to the hpra.. Non Prescription Labelling.
From www.slideserve.com
PPT Labelling of Certain Paediatric NonPrescription Cough and Cold Non Prescription Labelling Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Non Prescription Labelling.
From pharmac.govt.nz
Labelling preferences for medicines Pharmac New Zealand Government Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for. Non Prescription Labelling.
From www.youtube.com
How to read a medication label YouTube Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for. Non Prescription Labelling.
From www.fda.gov
OTC Drug Facts Label FDA Non Prescription Labelling General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Do not require prior notification to the. Non Prescription Labelling.
From karmelleven-platform.blogspot.com
Labeled Prescription With Doctors Name Sample Labelling Requirements Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients. Non Prescription Labelling.
From www.canada.ca
Labelling requirements for nonprescription drugs guidance document Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra.. Non Prescription Labelling.
From drugicon.cc
cvshealthpayorsolutionsscriptpathprescriptionlabelshelpmake Non Prescription Labelling Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling.. Non Prescription Labelling.
From www.canada.ca
Industry requirements for nonprescription drug labels Canada.ca Non Prescription Labelling Do not require prior notification to the hpra. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Non Prescription Labelling.
From www.umc.edu
Medication labels University of Mississippi Medical Center Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the. Non Prescription Labelling.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling.. Non Prescription Labelling.
From hellodoctor.com.ph
How to Read Drug Labels The Right Way A Guide Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best.. Non Prescription Labelling.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the hpra. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Non Prescription Labelling.
From medshadow.org
How to Read a Drug Label, According to a Pharmacist MedShadow Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra.. Non Prescription Labelling.
From www.slideserve.com
PPT Labelling of Certain Paediatric NonPrescription Cough and Cold Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. The european medicines agency has released draft quality review of documents recommendations on pack design and. Non Prescription Labelling.
From www.healthadvantage-hmo.com
How to read labels Health Advantage Non Prescription Labelling This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. General practice professionals should take steps to ensure. Non Prescription Labelling.
From pharmac.govt.nz
Labelling preferences for medicines Pharmac Te Pātaka Whaioranga Non Prescription Labelling Do not require prior notification to the hpra. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under. Non Prescription Labelling.
From www.healthywomen.org
How important is all the information on the labels of overthecounter Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. Do not require prior notification to the hpra. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that. Non Prescription Labelling.
From www.grc-health.com
Investigational Medicinal Product labelling an overview — GRCHealth Non Prescription Labelling The european medicines agency has released draft quality review of documents recommendations on pack design and labelling. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that patients are. Do not require prior notification to the hpra. Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a. Non Prescription Labelling.
From www.express-scripts.com
Understand your medication label Express Scripts® Pharmacy Non Prescription Labelling Advice on the legal labelling requirements for prescription only medicines and pharmacy medicines supplied under a patient. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Do not require prior notification to the hpra. General practice professionals should take steps to ensure that safe prescribing procedures are in place and that. Non Prescription Labelling.