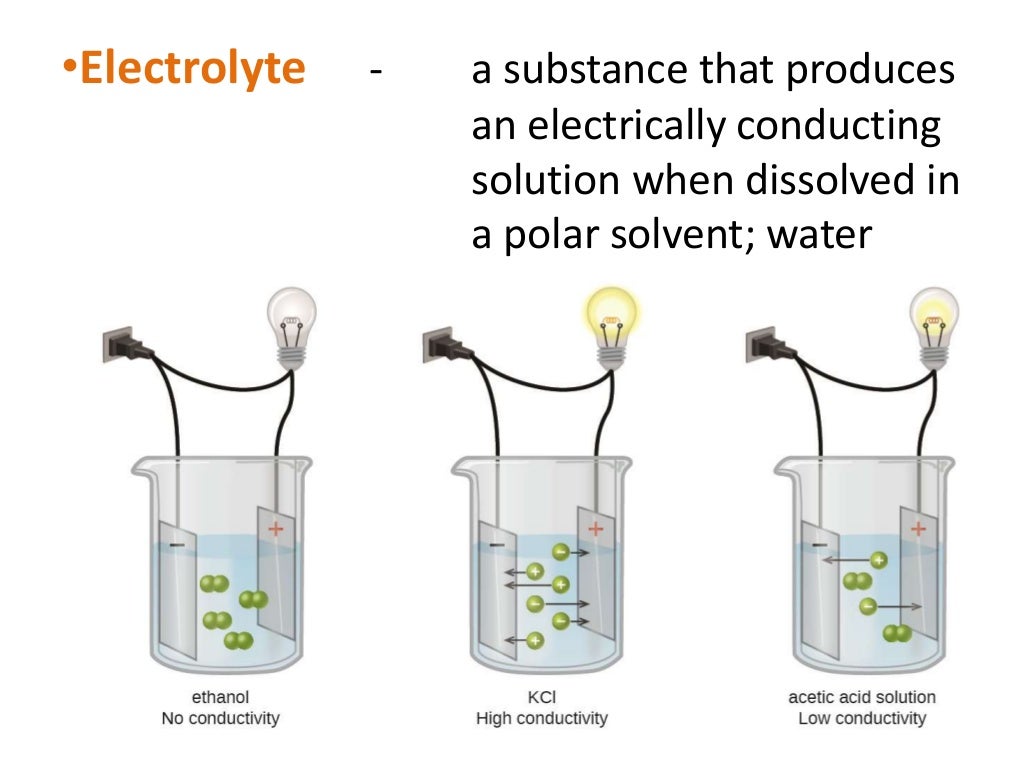
Electrochemical Cell Device Example . Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemistry is important in the transmission of nerve impulses in biological systems. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. Redox chemistry, the transfer of electrons, is behind all electrochemical processes.
from www.slideshare.net
An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemistry is important in the transmission of nerve impulses in biological systems. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy.
Electrochemistry electrochemical cells
Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is important in the transmission of nerve impulses in biological systems. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit.
From www.fuelcellstore.com
Basic Electrochemical Cell Setup Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemistry is important in the transmission of nerve impulses in biological systems. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell example of electrolytic cell usage is. Electrochemical Cell Device Example.
From www.asynt.com
Borosilicate electrochemical cells Asynt Electrochemical Cell Device Example An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.. Electrochemical Cell Device Example.
From www.slideshare.net
Electrochemistry electrochemical cells Electrochemical Cell Device Example This kind of cell includes the galvanic, or voltaic, cell, named after. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell example of electrolytic cell usage is electrolysis. Electrochemical Cell Device Example.
From www.researchgate.net
(A) Schematic diagram of the integrated scanning electrochemical cell Electrochemical Cell Device Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Redox. Electrochemical Cell Device Example.
From www.vecteezy.com
Electrochemical cell or Galvanic cell with Voltmeter and the function Electrochemical Cell Device Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Redox chemistry, the transfer of electrons, is behind all electrochemical. Electrochemical Cell Device Example.
From www.basinc.com
BASi® Electrochemical Cells Electrochemical Cell Device Example An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical. Electrochemical Cell Device Example.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Cell Device Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that produces an electric current from energy released. Electrochemical Cell Device Example.
From www.kingmariot.ae
Electrochemical cells & electroplating King Mariot Medical Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. Electrochemical cells, also known as galvanic cells or voltaic cells,. Electrochemical Cell Device Example.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrochemical Cell Device Example An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity. Electrochemical Cell Device Example.
From mmerevise.co.uk
Calculating the EMF Electrochemical Cell Device Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Electrochemical Cell Device Example.
From www.youtube.com
Lesson 19 Electrochemical Cell YouTube Electrochemical Cell Device Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. Electrochemical Cell Device Example.
From edu-rsc-org-ssl.oca.korea.ac.kr
Teach electrochemical cells using number grids Ideas RSC Education Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemistry is important in the transmission of nerve impulses in biological systems. Electrochemical cells, also. Electrochemical Cell Device Example.
From www.morebooks.de
Electrochemical Cell, 9786132668370, 6132668373 ,9786132668370 Electrochemical Cell Device Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. This kind. Electrochemical Cell Device Example.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemistry is important in the transmission of nerve impulses in biological systems. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells, also known as. Electrochemical Cell Device Example.
From www.basinc.com
BASi® Standard Electrochemical Cell (with 100 mL glass cell) Electrochemical Cell Device Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an. Electrochemical Cell Device Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical cells, also. Electrochemical Cell Device Example.
From studylib.net
Electrochemical cells Electrochemical Cell Device Example An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical. Electrochemical Cell Device Example.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An apparatus that is used to generate. Electrochemical Cell Device Example.
From saylordotorg.github.io
Electrochemistry Electrochemical Cell Device Example This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemical cells, also known as galvanic. Electrochemical Cell Device Example.
From redox.me
BE HCell 50 mL Basic Electrochemical HCell 50 mL affordable Electrochemical Cell Device Example This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An electrochemical cell is a device. Electrochemical Cell Device Example.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemical Cell Device Example An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Redox chemistry, the transfer of electrons, is behind all electrochemical processes.. Electrochemical Cell Device Example.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemistry is important in the transmission of nerve impulses in biological systems. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell example of electrolytic cell usage is. Electrochemical Cell Device Example.
From cn.gamry.com
Electrochemical Lithium Battery Test Equipment Cell Kit Electrochemical Cell Device Example Electrochemistry is important in the transmission of nerve impulses in biological systems. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Electrochemical cells, also known as galvanic cells or voltaic. Electrochemical Cell Device Example.
From learningcampusstall.z21.web.core.windows.net
Consider The Following Electrochemical Cell Electrochemical Cell Device Example An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. This kind of cell includes the galvanic, or voltaic, cell, named after. Redox chemistry, the transfer of electrons,. Electrochemical Cell Device Example.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Electrochemical Cell Device Example This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical cell splits the oxidant and reductant in a. Electrochemical Cell Device Example.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Electrochemical Cell Device Example An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells,. Electrochemical Cell Device Example.
From www.pinterest.com
Electrochemical Cells Chemwiki Chemistry Textbook, Gcse Chemistry Electrochemical Cell Device Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. An electrochemical cell is a device that produces an electric current from energy released. Electrochemical Cell Device Example.
From techxplore.com
Electrochemical device captures carbon dioxide at the flick of a switch Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. Electrochemistry is important in the transmission of nerve impulses in biological systems. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell example of electrolytic. Electrochemical Cell Device Example.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Cell Device Example An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. Electrochemistry is important in the transmission of. Electrochemical Cell Device Example.
From www.thoughtco.com
Electrochemical Cell EMF Example Problem Electrochemical Cell Device Example Electrochemistry is important in the transmission of nerve impulses in biological systems. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell example of electrolytic cell usage is electrolysis of water, and an example of a galvanic cell is a common. This kind of cell includes the galvanic,. Electrochemical Cell Device Example.
From www.vectorstock.com
Electrochemical cell or galvanic daniell Vector Image Electrochemical Cell Device Example Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell. Electrochemical Cell Device Example.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemical Cell Device Example Electrochemistry is important in the transmission of nerve impulses in biological systems. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Electrochemical cells,. Electrochemical Cell Device Example.
From www.basinc.com
BASi® Electrochemical Cells Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an. Redox chemistry, the transfer of electrons, is behind all electrochemical processes. An electrochemical. Electrochemical Cell Device Example.
From www.researchgate.net
1 Schematic diagram of the electrochemical cell [3] Download Electrochemical Cell Device Example This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a. Electrochemical Cell Device Example.
From studylib.net
3.ElectrochemicalCells Electrochemical Cell Device Example An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. This kind of cell includes the galvanic, or voltaic, cell, named after. Electrochemistry is important in the transmission of nerve impulses in biological systems. An electrochemical cell is a device that produces an electric current from energy released by a. Electrochemical Cell Device Example.