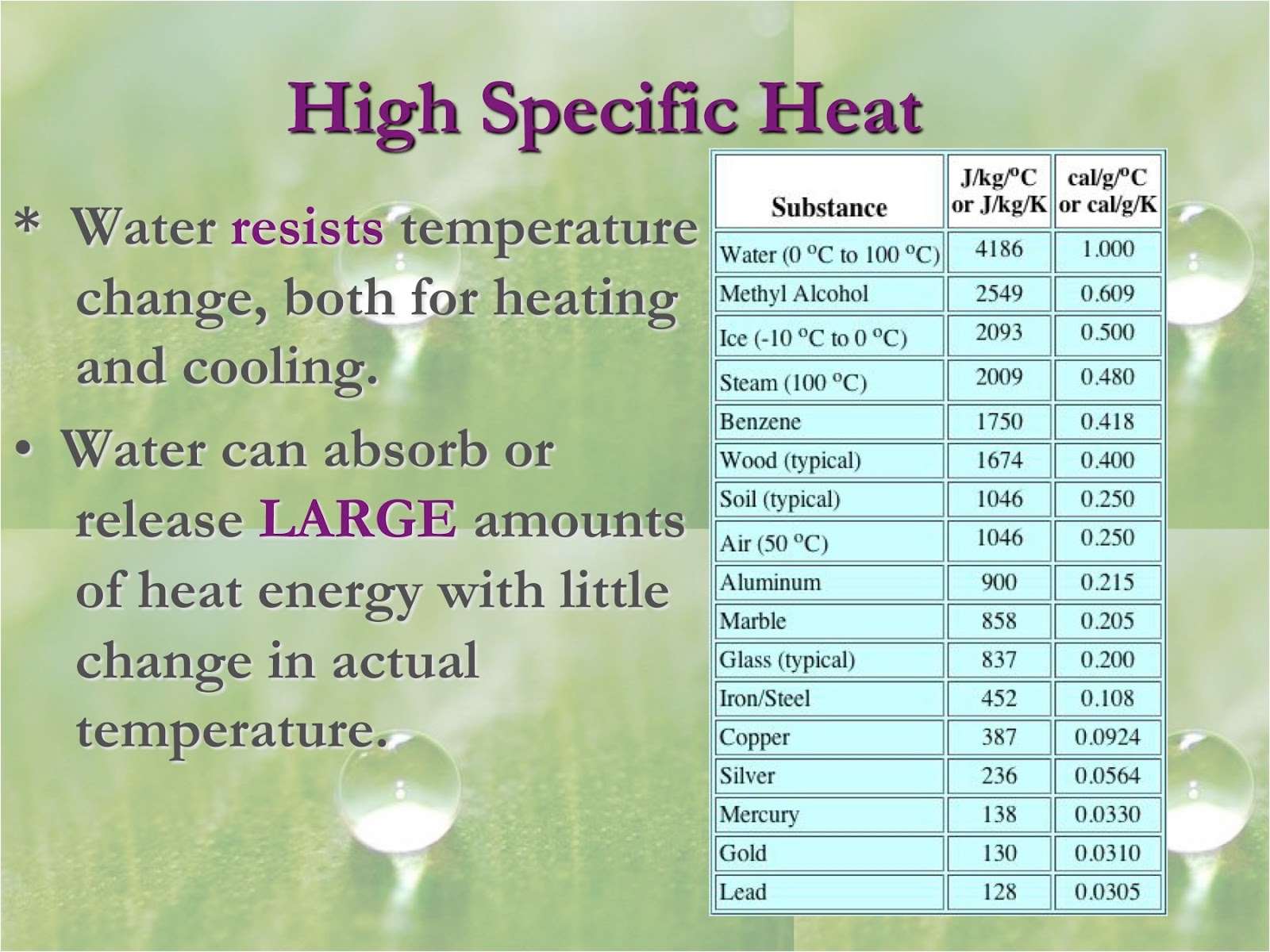
Why Is It Hard For Water To Rapidly Cool Or Heat Up . This property of water, its high heat capacity, is. You may not know how that affects. Water cools down and heats up at exactly the same rate under ideal conditions. As the temperature rises, the hydrogen bonds between water continually break and form anew. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. If you think about your experience with water, though, it makes sense. There is a very strong delta^+ on the. It therefore takes water a long time to heat and long time to cool. When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. In fact, the specific heat capacity of water is about five times more than that of sand. When heat is absorbed, hydrogen bonds are broken. The specific heat capacity of water is 4200 j/kg/c. Why does it take the water longer to warm up than either the air or the land? Consider the intermolecular forces between molecules of water. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules.
from missehonorsbio.blogspot.com
As the temperature rises, the hydrogen bonds between water continually break and form anew. The specific heat capacity of water is 4200 j/kg/c. This explains why the land. There is a very strong delta^+ on the. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. If you think about your experience with water, though, it makes sense. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. In fact, the specific heat capacity of water is about five times more than that of sand.
EC Honors Biology Specific Heat and Water as a Solvent
Why Is It Hard For Water To Rapidly Cool Or Heat Up When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. The specific heat capacity of water is 4200 j/kg/c. You may not know how that affects. As the temperature rises, the hydrogen bonds between water continually break and form anew. There is a very strong delta^+ on the. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. In fact, the specific heat capacity of water is about five times more than that of sand. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. This property of water, its high heat capacity, is. If you think about your experience with water, though, it makes sense. This explains why the land. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. Water cools down and heats up at exactly the same rate under ideal conditions. Water can absorb a very large amount of heat and only a small increase in its overall temperature. Why does it take the water longer to warm up than either the air or the land? Consider the intermolecular forces between molecules of water.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2956417 Why Is It Hard For Water To Rapidly Cool Or Heat Up It therefore takes water a long time to heat and long time to cool. As the temperature rises, the hydrogen bonds between water continually break and form anew. Water cools down and heats up at exactly the same rate under ideal conditions. You may not know how that affects. If you think about your experience with water, though, it makes. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From blog.constellation.com
Hard vs. Soft Water Which Is Better? Constellation Why Is It Hard For Water To Rapidly Cool Or Heat Up As the temperature rises, the hydrogen bonds between water continually break and form anew. In fact, the specific heat capacity of water is about five times more than that of sand. If you think about your experience with water, though, it makes sense. It therefore takes water a long time to heat and long time to cool. Water has a. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From waternitylab.com
Water Hardness Scale GPG, mmol/L, PPM Chart Why Is It Hard For Water To Rapidly Cool Or Heat Up Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. Water cools down and heats up at exactly the same rate under ideal conditions. Water can absorb a very large amount of heat and only a small increase in its overall temperature. Why does it take the water longer to warm up. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From stock.adobe.com
Convection process diagram. Warm air rises and cool air sinks. Hot and Why Is It Hard For Water To Rapidly Cool Or Heat Up Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Water cools down and heats up at exactly the same rate under ideal conditions. Water can absorb a very large amount of heat and only a small increase in its overall temperature. There is a very strong delta^+ on the. Consider the intermolecular forces between molecules. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.iqsdirectory.com
Water Chiller What Is It? How Does It Work? Types, Uses Why Is It Hard For Water To Rapidly Cool Or Heat Up Why does it take the water longer to warm up than either the air or the land? As the temperature rises, the hydrogen bonds between water continually break and form anew. This explains why the land. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. The specific heat capacity of water. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Why Is It Hard For Water To Rapidly Cool Or Heat Up As the temperature rises, the hydrogen bonds between water continually break and form anew. The specific heat capacity of water is 4200 j/kg/c. It therefore takes water a long time to heat and long time to cool. When heat is absorbed, hydrogen bonds are broken. You may not know how that affects. Water has a high specific heat capacity—it absorbs. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Why Is It Hard For Water To Rapidly Cool Or Heat Up When heat is absorbed, hydrogen bonds are broken. When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. The specific heat capacity of water is 4200 j/kg/c. This property. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From exorqgpiu.blob.core.windows.net
What Is A Water Cooled Computer at Samual Bird blog Why Is It Hard For Water To Rapidly Cool Or Heat Up This explains why the land. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. There is a very strong delta^+ on the. You may not know how that. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From newatersofteners.co.uk
Hard Water Why You Need Soft Water NE Water Softeners Why Is It Hard For Water To Rapidly Cool Or Heat Up There is a very strong delta^+ on the. In fact, the specific heat capacity of water is about five times more than that of sand. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. When the sun is shining bright, it seems like the water should be as inviting as the. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Why Is It Hard For Water To Rapidly Cool Or Heat Up In fact, the specific heat capacity of water is about five times more than that of sand. You may not know how that affects. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. Consider the intermolecular forces between molecules of water. It. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From hompros.com
How Does a Water Cooler Work Hompros Why Is It Hard For Water To Rapidly Cool Or Heat Up If you think about your experience with water, though, it makes sense. It therefore takes water a long time to heat and long time to cool. This explains why the land. You may not know how that affects. Why does it take the water longer to warm up than either the air or the land? When heat is absorbed, hydrogen. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From mavink.com
Formula Of Specific Heat Capacity Why Is It Hard For Water To Rapidly Cool Or Heat Up There is a very strong delta^+ on the. This explains why the land. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. This property of water, its high heat capacity, is. The specific heat capacity of water is 4200 j/kg/c. As the. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.sciencefacts.net
Water Expansion When Freezing Why Is It Hard For Water To Rapidly Cool Or Heat Up Water can absorb a very large amount of heat and only a small increase in its overall temperature. As the temperature rises, the hydrogen bonds between water continually break and form anew. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. You. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From ch301.cm.utexas.edu
heating curve Why Is It Hard For Water To Rapidly Cool Or Heat Up You may not know how that affects. It therefore takes water a long time to heat and long time to cool. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. This explains why the land. Consider the intermolecular forces between molecules of water. As the temperature rises, the hydrogen bonds between. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From purewaterblog.com
Is Distilled Water Hard or Soft? Soft Water vs. Hard Water Water Why Is It Hard For Water To Rapidly Cool Or Heat Up Water cools down and heats up at exactly the same rate under ideal conditions. If you think about your experience with water, though, it makes sense. Consider the intermolecular forces between molecules of water. This explains why the land. The specific heat capacity of water is 4200 j/kg/c. In fact, the specific heat capacity of water is about five times. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From habr.com
Долгая история реакторов на быстрых нейтронах и обещания использования Why Is It Hard For Water To Rapidly Cool Or Heat Up Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. As the temperature rises, the hydrogen bonds between water continually break and form anew. There is a very strong delta^+ on the. This property of water, its high heat capacity, is. You may not know how that affects. Water has a high specific heat capacity—it absorbs. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.aplustopper.com
How does the Temperature Affect the Movement of Particles A Plus Topper Why Is It Hard For Water To Rapidly Cool Or Heat Up Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. There is a very strong delta^+ on the. In fact, the specific heat capacity of water is about five times more than that of sand. You may not know how that affects. Water cools down and heats up at exactly the same. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center Why Is It Hard For Water To Rapidly Cool Or Heat Up As the temperature rises, the hydrogen bonds between water continually break and form anew. You may not know how that affects. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. If you think about your experience with water, though, it makes sense.. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From exoyoxocc.blob.core.windows.net
Dual Pump Water Cooling Loop at Rose Silvis blog Why Is It Hard For Water To Rapidly Cool Or Heat Up Water cools down and heats up at exactly the same rate under ideal conditions. Water can absorb a very large amount of heat and only a small increase in its overall temperature. If you think about your experience with water, though, it makes sense. When heat is absorbed, hydrogen bonds are broken. It therefore takes water a long time to. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From arenahanna.wordpress.com
Transfer processing of heat energy HEAT WORLD OF PHYSICS Why Is It Hard For Water To Rapidly Cool Or Heat Up The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. You may not know how that affects. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Why does it take the water longer to warm up than either. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From hxebiuqww.blob.core.windows.net
Water Cooling System Temperature at Leslie Pace blog Why Is It Hard For Water To Rapidly Cool Or Heat Up Consider the intermolecular forces between molecules of water. When heat is absorbed, hydrogen bonds are broken. This property of water, its high heat capacity, is. This explains why the land. As the temperature rises, the hydrogen bonds between water continually break and form anew. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Is It Hard For Water To Rapidly Cool Or Heat Up Consider the intermolecular forces between molecules of water. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. In fact, the specific heat capacity of water is about five times more than that of sand. You may not. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.thespruce.com
Hard Water and How It Damages Plumbing Why Is It Hard For Water To Rapidly Cool Or Heat Up In fact, the specific heat capacity of water is about five times more than that of sand. When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. There is a very strong delta^+ on the. The specific heat capacity of water is 4200 j/kg/c. Water’s high heat. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From jmpcoblog.com
Introduction to Water Source Heat Pump Systems Part 3 Basic Operation Why Is It Hard For Water To Rapidly Cool Or Heat Up This property of water, its high heat capacity, is. Water cools down and heats up at exactly the same rate under ideal conditions. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. Water has a high specific heat capacity—it absorbs a lot. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From hvactrainingshop.com
How a Chilled Water System Works HVAC Training Shop Why Is It Hard For Water To Rapidly Cool Or Heat Up The specific heat capacity of water is 4200 j/kg/c. Water can absorb a very large amount of heat and only a small increase in its overall temperature. This property of water, its high heat capacity, is. There is a very strong delta^+ on the. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From h2ocare.com
Hard Water Infographic H2O Care Why Is It Hard For Water To Rapidly Cool Or Heat Up There is a very strong delta^+ on the. Water cools down and heats up at exactly the same rate under ideal conditions. This property of water, its high heat capacity, is. You may not know how that affects. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. When heat is absorbed,. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From slideplayer.com
Seasons and Atmosphere ppt download Why Is It Hard For Water To Rapidly Cool Or Heat Up The specific heat capacity of water is 4200 j/kg/c. In fact, the specific heat capacity of water is about five times more than that of sand. Water cools down and heats up at exactly the same rate under ideal conditions. This explains why the land. Water can absorb a very large amount of heat and only a small increase in. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From scaleblaster.com
Understanding Hard Water and Its Effects Why Is It Hard For Water To Rapidly Cool Or Heat Up As the temperature rises, the hydrogen bonds between water continually break and form anew. Why does it take the water longer to warm up than either the air or the land? When heat is absorbed, hydrogen bonds are broken. Consider the intermolecular forces between molecules of water. Water’s high heat capacity is a property caused by hydrogen bonding among water. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From exoecqyjo.blob.core.windows.net
Coolant And Water at Letitia Delfino blog Why Is It Hard For Water To Rapidly Cool Or Heat Up As the temperature rises, the hydrogen bonds between water continually break and form anew. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. This property of water, its high heat capacity, is. In fact, the specific heat capacity of water is about five times more than that of sand. Consider the. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From socratic.org
How can heat cause motion in a liquid? + Example Why Is It Hard For Water To Rapidly Cool Or Heat Up In fact, the specific heat capacity of water is about five times more than that of sand. When heat is absorbed, hydrogen bonds are broken. This property of water, its high heat capacity, is. As the temperature rises, the hydrogen bonds between water continually break and form anew. Water has a high specific heat capacity—it absorbs a lot of heat. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From missehonorsbio.blogspot.com
EC Honors Biology Specific Heat and Water as a Solvent Why Is It Hard For Water To Rapidly Cool Or Heat Up This explains why the land. If you think about your experience with water, though, it makes sense. Water cools down and heats up at exactly the same rate under ideal conditions. It therefore takes water a long time to heat and long time to cool. Why does it take the water longer to warm up than either the air or. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From exozvyfdp.blob.core.windows.net
Water Cooled Chiller Technical Data Sheet at Lynn Anderson blog Why Is It Hard For Water To Rapidly Cool Or Heat Up Why does it take the water longer to warm up than either the air or the land? The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. If you think about your experience with water, though, it makes sense. When heat is absorbed,. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Why Is It Hard For Water To Rapidly Cool Or Heat Up This explains why the land. There is a very strong delta^+ on the. When the sun is shining bright, it seems like the water should be as inviting as the air and the land around you. It therefore takes water a long time to heat and long time to cool. This property of water, its high heat capacity, is. Water. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From purewaterblog.com
Real World Examples of Hard Water Water Treatment Why Is It Hard For Water To Rapidly Cool Or Heat Up You may not know how that affects. As the temperature rises, the hydrogen bonds between water continually break and form anew. This property of water, its high heat capacity, is. Water cools down and heats up at exactly the same rate under ideal conditions. Consider the intermolecular forces between molecules of water. There is a very strong delta^+ on the.. Why Is It Hard For Water To Rapidly Cool Or Heat Up.
From sciencenotes.org
Hard Water vs Soft Water Know the Difference Why Is It Hard For Water To Rapidly Cool Or Heat Up Water cools down and heats up at exactly the same rate under ideal conditions. If you think about your experience with water, though, it makes sense. The hydrogen bonds between water molecules cause the water to have a high heat capacity, meaning it takes a lot of added heat to raise its temperature. This explains why the land. When heat. Why Is It Hard For Water To Rapidly Cool Or Heat Up.