How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction . calorimetry and enthalpies of reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the.
from general.chemistrysteps.com
To do so, the heat is exchanged with a calibrated object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which measure the. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and enthalpies of reaction. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. To do so, the heat is exchanged with a.
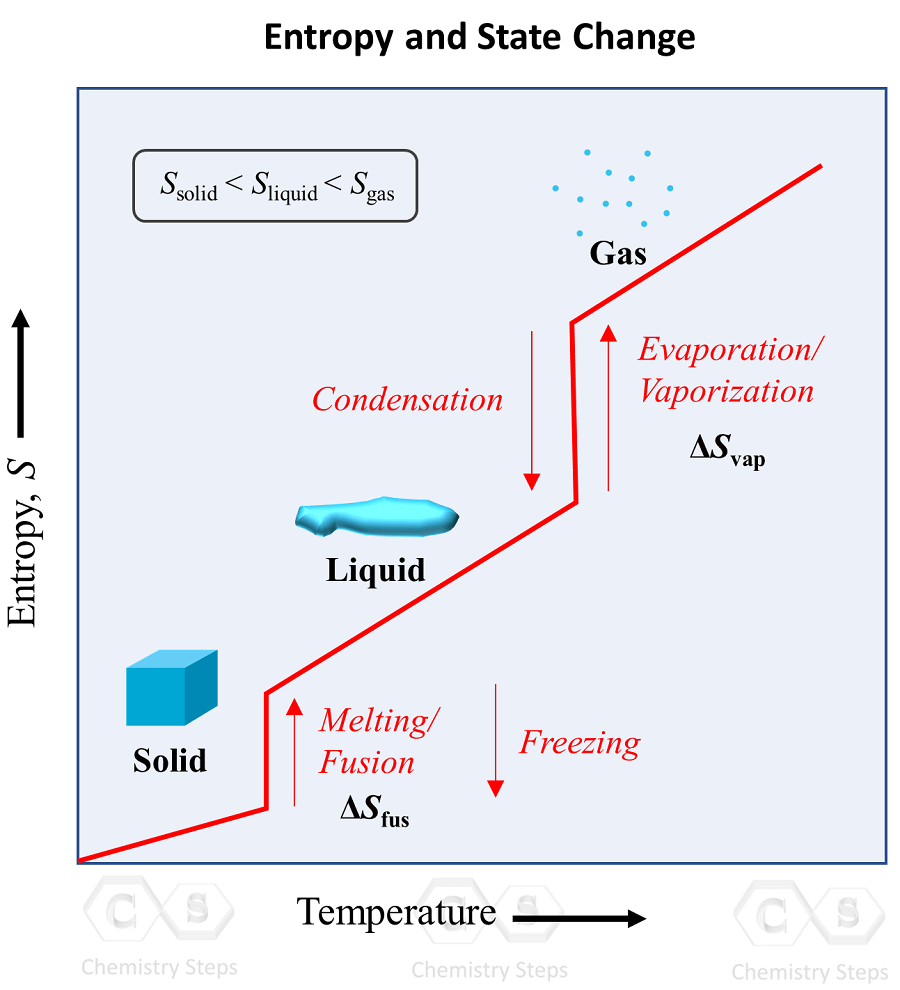
Entropy and State Change Chemistry Steps
How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry and enthalpies of reaction. To do so, the heat is exchanged with a calibrated object. It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and enthalpies of reaction. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry and enthalpies of reaction. It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters can also measure. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. . How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. calorimetry. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From users.highland.edu
Calorimetry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and enthalpies of reaction. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. measuring the enthalpy change for a. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Measuring Enthalpy Changes PowerPoint Presentation, free download How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. measuring the enthalpy change. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. It uses devices called. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the. measuring the enthalpy change. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is the set of techniques used to measure enthalpy. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which measure the. calorimetry is used to measure amounts of heat transferred to or from a. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.youtube.com
Measuring Enthalpy Changes Using The Calorimeter YouTube How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry and enthalpies of reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a. in this explainer, we will learn how to perform calorimetry experiments and. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry and enthalpies of reaction. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. measuring the enthalpy change. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From dxoqgiaho.blob.core.windows.net
Bomb Calorimeter Enthalpy Of Combustion at Harold Shaner blog How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry and enthalpies of reaction. It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Chemical Energetics (5) Measuring the enthalpy change of How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry and enthalpies of reaction. It uses devices called calorimeters, which measure the. Calorimeters can also measure the molar enthalpy of reaction, by simply equating. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and enthalpies of reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. To. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.linstitute.net
AQA A Level Chemistry复习笔记4.1.3 Measurement of an Enthalpy Change翰林国际教育 How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry and enthalpies of reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. calorimetry and enthalpies of reaction. It uses devices called calorimeters, which measure the. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry and enthalpies of reaction. Calorimeters can also measure the molar enthalpy of reaction, by. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Enthalpy Changes PowerPoint Presentation ID1551541 How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction It uses devices called calorimeters, which measure the. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Chemistry The Molecular Science Moore, Stanitski and Jurs How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. To do so, the heat is exchanged with a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. measuring the enthalpy change for a reaction. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Measuring Enthalpy Changes PowerPoint Presentation, free download How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. in this explainer, we will learn how to perform calorimetry experiments and. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From thechemistrynotes.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry and enthalpies of reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is used to measure amounts of heat transferred to or from a substance. in this explainer, we will learn how to perform calorimetry experiments. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which measure the. measuring. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Measuring Enthalpy Changes PowerPoint Presentation, free download How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry and enthalpies of reaction. calorimetry is used. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction To do so, the heat is exchanged with a calibrated object. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.gauthmath.com
Solved Targ 4) Select 3 that apply. How does a calorimeter measure the How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. measuring the enthalpy change for a reaction experimentally calorimetric method if the reaction is slow then the. . How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which measure the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.
From ihsanpedia.com
How To Calculate Enthalpy Change A Comprehensive Guide IHSANPEDIA How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. calorimetry is used to measure amounts of heat transferred to or from a substance. It uses devices called calorimeters, which measure. How Does A Calorimeter Measure The Enthalpy Change Of A Chemical Reaction.