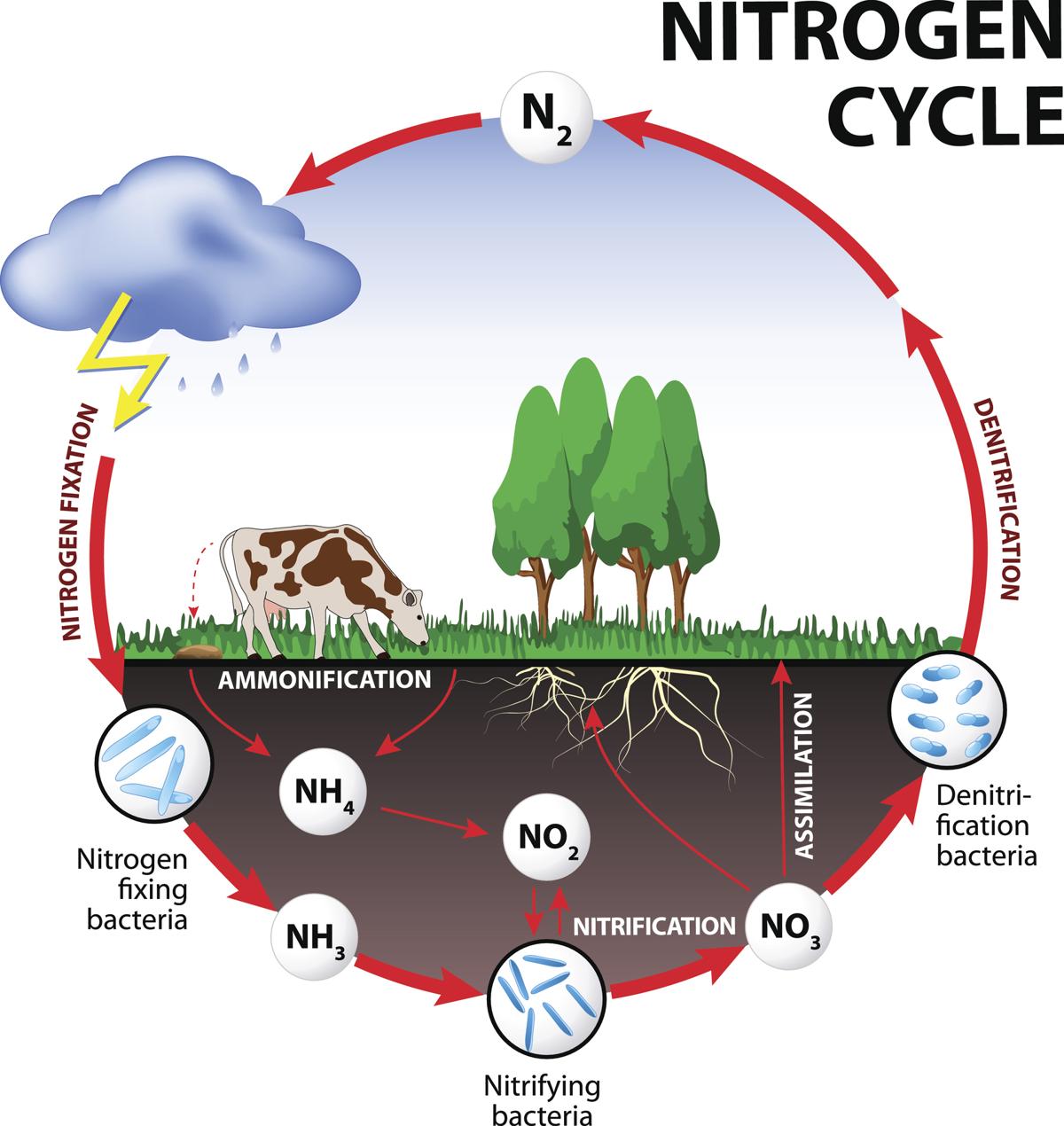
What Is Nitrogen Fixation Easy Definition . A simple definition of nitrogen fixation. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. Nitrogen is taken up by plants from the soil and used for protein synthesis. 3) or other nitrogenous compounds in the soil. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain.
from www.onlinebiologynotes.com
In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. A simple definition of nitrogen fixation. Nitrogen is taken up by plants from the soil and used for protein synthesis. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain.
Nitrogen cycle Steps of Nitrogen cycle Online Biology Notes
What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. Nitrogen is taken up by plants from the soil and used for protein synthesis. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. A simple definition of nitrogen fixation. 3) or other nitrogenous compounds in the soil.
From www.slideshare.net
NITROGEN CYCLE AND FIXING What Is Nitrogen Fixation Easy Definition 3) or other nitrogenous compounds in the soil. A simple definition of nitrogen fixation. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in. What Is Nitrogen Fixation Easy Definition.
From www.phdnest.com
Nitrogen Metabolism Nitrogen Cycle Daigram, Biological Fixation What Is Nitrogen Fixation Easy Definition The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. 3) or other nitrogenous compounds in the soil. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. A simple definition of. What Is Nitrogen Fixation Easy Definition.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation ID7047601 What Is Nitrogen Fixation Easy Definition Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. 3). What Is Nitrogen Fixation Easy Definition.
From byjus.com
Nitrogen Cycle Explained Definition, Stages and Importance What Is Nitrogen Fixation Easy Definition A simple definition of nitrogen fixation. Nitrogen is taken up by plants from the soil and used for protein synthesis. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air. What Is Nitrogen Fixation Easy Definition.
From gamesmartz.com
Nitrogen Fixation Definition & Image GameSmartz What Is Nitrogen Fixation Easy Definition Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. 3) or other nitrogenous compounds in the soil. A simple definition of nitrogen fixation. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3). What Is Nitrogen Fixation Easy Definition.
From ar.inspiredpencil.com
Nitrogen Fixation Definition What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation. What Is Nitrogen Fixation Easy Definition.
From www.slideshare.net
Nitrogen fixation mechanism in legumes What Is Nitrogen Fixation Easy Definition Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. 3) or other nitrogenous compounds in the soil. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which. What Is Nitrogen Fixation Easy Definition.
From www.tutoroot.com
What is Nitrogen Cycle? Diagram, Stages, Importance Tutoroot What Is Nitrogen Fixation Easy Definition Nitrogen is taken up by plants from the soil and used for protein synthesis. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. In contrast, nitrogen fixation is the process by which atmospheric. What Is Nitrogen Fixation Easy Definition.
From scienceinfo.com
Nitrogen Cycle Definition, 5 Cycle Steps, Importance What Is Nitrogen Fixation Easy Definition A simple definition of nitrogen fixation. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. 3) or other nitrogenous compounds in the soil.. What Is Nitrogen Fixation Easy Definition.
From exywqxyjf.blob.core.windows.net
What Is Nitrogen Fixation A Level Biology at Ruthie Tan blog What Is Nitrogen Fixation Easy Definition Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. In contrast, nitrogen fixation is the process by which atmospheric nitrogen. What Is Nitrogen Fixation Easy Definition.
From plantsandpipettes.com
Can Scientists Hack Nitrogen Fixation? Plants and Pipettes What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2). What Is Nitrogen Fixation Easy Definition.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn What Is Nitrogen Fixation Easy Definition 3) or other nitrogenous compounds in the soil. The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in. What Is Nitrogen Fixation Easy Definition.
From www.slideserve.com
PPT NITROGEN FIXATION PowerPoint Presentation, free download ID4437387 What Is Nitrogen Fixation Easy Definition 3) or other nitrogenous compounds in the soil. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air. What Is Nitrogen Fixation Easy Definition.
From www.sciencefacts.net
Nitrogen Fixation Definition, Process, Example & Equation What Is Nitrogen Fixation Easy Definition It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in. What Is Nitrogen Fixation Easy Definition.
From www.sciencefacts.net
Nitrogen Cycle Definition, Steps, Importance with Diagram What Is Nitrogen Fixation Easy Definition 3) or other nitrogenous compounds in the soil. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). A simple definition of nitrogen fixation. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated. What Is Nitrogen Fixation Easy Definition.
From www.thetreecenter.com
What is Nitrogen Fixation The Tree Center™ What Is Nitrogen Fixation Easy Definition It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. The conversion of atmospheric nitrogen into. What Is Nitrogen Fixation Easy Definition.
From www.geeksforgeeks.org
Nitrogen Fixation Definition, Types, Properties, Examples, FAQs What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that. What Is Nitrogen Fixation Easy Definition.
From www.toppr.com
Nitrogen Cycle Definition, Steps, Importance and Solved Example What Is Nitrogen Fixation Easy Definition 3) or other nitrogenous compounds in the soil. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. In contrast, nitrogen. What Is Nitrogen Fixation Easy Definition.
From in.pinterest.com
What is nitrogen cycle By Unacademy Nitrogen cycle, Nitrogen fixation What Is Nitrogen Fixation Easy Definition In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. A. What Is Nitrogen Fixation Easy Definition.
From www.priyamstudycentre.com
Nitrogen Fixation Definition, Process, Examples What Is Nitrogen Fixation Easy Definition In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into. What Is Nitrogen Fixation Easy Definition.
From www.toppr.com
The given diagram shows a part of the nitrogen cycle. Which of the What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen is taken up by plants from the soil and used for protein synthesis. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. 3) or other nitrogenous compounds in the soil. Nitrogen fixation is a chemical process in which. What Is Nitrogen Fixation Easy Definition.
From ar.inspiredpencil.com
Nitrogen Fixation Diagram What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. 3) or other nitrogenous compounds in the soil. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas. What Is Nitrogen Fixation Easy Definition.
From gardentabs.com
How Do Plants Use Nitrogen? What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen fixation is a chemical phenomenon. What Is Nitrogen Fixation Easy Definition.
From exyyymgle.blob.core.windows.net
What Is The Importance In Nature Of Recycling Carbon And Nitrogen at What Is Nitrogen Fixation Easy Definition Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Nitrogen. What Is Nitrogen Fixation Easy Definition.
From www.onlinebiologynotes.com
Nitrogen cycle Steps of Nitrogen cycle Online Biology Notes What Is Nitrogen Fixation Easy Definition Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in. What Is Nitrogen Fixation Easy Definition.
From www.vedantu.com
What is meant by nitrogen fixation? Explain biological nitrogen What Is Nitrogen Fixation Easy Definition Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. 3) or other nitrogenous compounds in the soil. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia. What Is Nitrogen Fixation Easy Definition.
From www.britannica.com
nitrogen fixation Definition, Process, Examples, Types, & Facts What Is Nitrogen Fixation Easy Definition Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh 3). Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. Nitrogen fixation is how. What Is Nitrogen Fixation Easy Definition.
From ar.inspiredpencil.com
Nitrogen Fixation Definition What Is Nitrogen Fixation Easy Definition The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia. What Is Nitrogen Fixation Easy Definition.
From ar.inspiredpencil.com
Simple Nitrogen Fixation What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. Nitrogen fixation converts or ‘fixes’ nitrogen into a form organisms can use. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is. What Is Nitrogen Fixation Easy Definition.
From www.adda247.com
Nitrogen Cycle Diagram, Steps, Drawing for Class 8 & 9 What Is Nitrogen Fixation Easy Definition Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen fixation is a chemical phenomenon involving the conversion of molecular nitrogen present in the atmosphere into ammonia or associated nitrogenous. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is fixed by natural or industrial means to form ammonia (nh. What Is Nitrogen Fixation Easy Definition.
From www.vedantu.com
Describe the nitrogen cycle with the help of a diagram. What Is Nitrogen Fixation Easy Definition Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. In contrast, nitrogen fixation is the process by which atmospheric nitrogen gas (n 2) is. What Is Nitrogen Fixation Easy Definition.
From www.drishtiias.com
Nitrogen Pollution What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. Nitrogen is taken up by plants from the soil and used for protein synthesis. Nitrogen fixation is how nitrogen in the air (n 2) is changed (converted) into ammonia (nh. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert.. What Is Nitrogen Fixation Easy Definition.
From scienceinfo.com
Nitrogen Fixation Definition, Methods, and Benefits What Is Nitrogen Fixation Easy Definition Nitrogen present in these nitrogenous compounds is called fixed or combined nitrogen. 3) or other nitrogenous compounds in the soil. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. A simple definition of nitrogen fixation. The. What Is Nitrogen Fixation Easy Definition.
From www.britannica.com
nitrogen fixation Definition, Process, Examples, Types, & Facts What Is Nitrogen Fixation Easy Definition A simple definition of nitrogen fixation. Aside from a few microbes, atmospheric nitrogen is molecular dinitrogen, a generally nonreactive chemical that is biologically inert. It is the conversion of atmospheric nitrogen (n 2) into ammonia (nh 3) or related nitrogenous compounds, which are assimilated by plants and subsequently enter the food chain. In contrast, nitrogen fixation is the process by. What Is Nitrogen Fixation Easy Definition.
From www.carmeninthegarden.com
How Nitrogen Fixation Works in Your Garden Carmen in the Garden What Is Nitrogen Fixation Easy Definition The conversion of atmospheric nitrogen into useful nitrogenous compounds, by natural or artificial methods, is called the fixation of nitrogen. Nitrogen fixation is a chemical process in which molecular nitrogen (n2) in the air is transformed into ammonia (nh3) or related nitrogenous chemicals, mainly in soil or aquatic environments but also in industry. Nitrogen fixation is a chemical phenomenon involving. What Is Nitrogen Fixation Easy Definition.