Brittle Chemical Bond . this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. (i) covalent bond crystals are usually hard and brittle; (ii) binding energy is high so that their melting and boiling. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; a bond is an electrostatic force that holds the atoms of elements together in a compound. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. There are three types of. Covalent bonding arises from the sharing of two or more electrons between atoms.
from www.worksheetsplanet.com
(i) covalent bond crystals are usually hard and brittle; since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. a bond is an electrostatic force that holds the atoms of elements together in a compound. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (ii) binding energy is high so that their melting and boiling. Covalent bonding arises from the sharing of two or more electrons between atoms. There are three types of. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials.
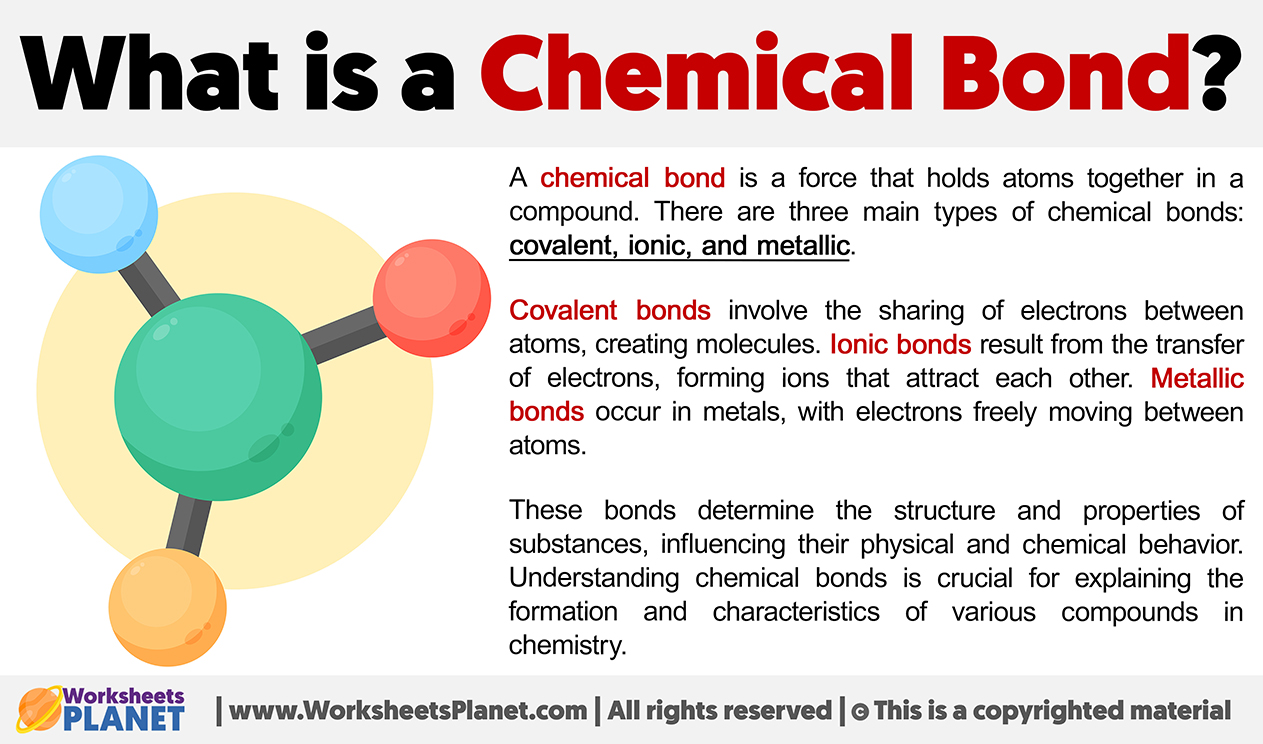
What is a Chemical Bond
Brittle Chemical Bond There are three types of. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. a bond is an electrostatic force that holds the atoms of elements together in a compound. (ii) binding energy is high so that their melting and boiling. Covalent bonding arises from the sharing of two or more electrons between atoms. There are three types of. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. (i) covalent bond crystals are usually hard and brittle; since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond.
From slideplayer.com
Chemical Bonding. ppt download Brittle Chemical Bond Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (i) covalent bond crystals are usually hard and brittle; this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials.. Brittle Chemical Bond.
From www.researchgate.net
Schematic illustration of the mechanism of brittleductile transition Brittle Chemical Bond the bonding types are called electrovalent (or ionic) bonding and covalent bonding. this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. There are three types of. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Solid materials can be categorized based on their. Brittle Chemical Bond.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding PowerPoint Presentation, free download Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (i) covalent bond crystals are usually hard and brittle;. Brittle Chemical Bond.
From slidetodoc.com
UNIT 3 NOTES EDGENUITY CHEMICAL BONDING CHEMICAL BOND Brittle Chemical Bond Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Covalent bonding arises from the sharing of two or more electrons between atoms. There are three types of. (ii) binding energy is high so that their melting and boiling. this review covers the essential features of crack behavior in characteristically brittle solids, starting. Brittle Chemical Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID1587654 Brittle Chemical Bond the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a. Brittle Chemical Bond.
From slideplayer.com
Chapter 6 Chemical Bonding. ppt download Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. (ii) binding energy is high so that their melting and boiling. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (i) covalent bond crystals are usually hard and brittle; since inherent brittleness is. Brittle Chemical Bond.
From www.chemistry-teaching-resources.com
chemistry picture Brittle Chemical Bond a bond is an electrostatic force that holds the atoms of elements together in a compound. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. this review covers the essential features of crack behavior in. Brittle Chemical Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID1587654 Brittle Chemical Bond Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; a bond is an electrostatic force that holds the atoms of elements together in a compound. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. There are three types of. (ii) binding energy is high so that their melting. Brittle Chemical Bond.
From www.slideserve.com
PPT BONDING & NOMENCLATURE PowerPoint Presentation, free download Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. a bond is an electrostatic force that holds the atoms of elements together in a compound. There are three types of. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (ii) binding energy is high so that their melting. Brittle Chemical Bond.
From www.coolkidfacts.com
Chemical Bonding Facts Chemistry Cool Kid Facts Brittle Chemical Bond since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. a bond is an electrostatic force that holds the atoms of elements together in a compound. There are three types of. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; this review covers the. Brittle Chemical Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID1587654 Brittle Chemical Bond There are three types of. this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. (i) covalent bond crystals are usually hard and brittle; Electrovalent bonding arises from complete transfer of one or more electrons. Brittle Chemical Bond.
From impressedselect.blogspot.com
which hybridization scheme allows the formation of at least one π bond Brittle Chemical Bond since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. (i) covalent bond crystals are usually hard and brittle; (ii) binding energy is high so that their melting and boiling. Covalent bonding arises from the sharing of two or. Brittle Chemical Bond.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. (i) covalent bond crystals are usually hard and brittle; the bonding types are called electrovalent (or ionic) bonding and covalent bonding. There are three types of. a bond is an electrostatic force that holds the atoms of elements together. Brittle Chemical Bond.
From slideplayer.com
Chemical Bonding Chapter 8 ppt download Brittle Chemical Bond (i) covalent bond crystals are usually hard and brittle; this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. There are three types of. (ii) binding energy is high so that their melting and. Brittle Chemical Bond.
From slideplayer.com
Chemical Bonding. ppt download Brittle Chemical Bond There are three types of. (ii) binding energy is high so that their melting and boiling. a bond is an electrostatic force that holds the atoms of elements together in a compound. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. this review covers the essential features of crack behavior in characteristically brittle solids,. Brittle Chemical Bond.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Covalent bonding arises from the sharing of two or more electrons between atoms. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. (ii) binding energy is high so that their melting and boiling. the. Brittle Chemical Bond.
From www.britannica.com
Bodycentred cubic structure crystalline form Britannica Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. (ii) binding energy is high so that their melting and boiling. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; (i) covalent bond crystals are usually. Brittle Chemical Bond.
From www.biorender.com
Types of Chemical Bonds BioRender Science Templates Brittle Chemical Bond Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Solid materials can be categorized based on their behavior under loading into brittle. Brittle Chemical Bond.
From www.researchgate.net
(a) Stress/strain relationships for brittle, plastic, and elastomeric Brittle Chemical Bond a bond is an electrostatic force that holds the atoms of elements together in a compound. There are three types of. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. since inherent brittleness is suspected. Brittle Chemical Bond.
From slideplayer.com
CHEMICAL BONDING _____ BONDS. ppt download Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. There are three types of. a bond is an electrostatic force that holds the atoms of elements together in a compound. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; Solid materials can be. Brittle Chemical Bond.
From www.slideserve.com
PPT Unit 4 Metallic Bonding PowerPoint Presentation, free download Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. (i) covalent bond crystals are usually hard and brittle; the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Solid materials can be categorized based on. Brittle Chemical Bond.
From slideplayer.com
Chemical Bonds and Compounds ppt download Brittle Chemical Bond this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. Covalent bonding arises from the sharing of two or more electrons between atoms. a bond is an electrostatic force that holds the atoms of elements. Brittle Chemical Bond.
From www.slideshare.net
Chemical bonding Brittle Chemical Bond (i) covalent bond crystals are usually hard and brittle; a bond is an electrostatic force that holds the atoms of elements together in a compound. (ii) binding energy is high so that their melting and boiling. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. Electrovalent bonding arises from complete transfer. Brittle Chemical Bond.
From printablejuliocbgov.z21.web.core.windows.net
Types Of Chemical Bonds Chemistry Brittle Chemical Bond (ii) binding energy is high so that their melting and boiling. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Covalent bonding arises from the sharing of two or more electrons between atoms. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. There are three types of. Solid. Brittle Chemical Bond.
From www.researchgate.net
The correlation between ductile/brittle and types of bond. Au is Brittle Chemical Bond (ii) binding energy is high so that their melting and boiling. (i) covalent bond crystals are usually hard and brittle; Covalent bonding arises from the sharing of two or more electrons between atoms. a bond is an electrostatic force that holds the atoms of elements together in a compound. Electrovalent bonding arises from complete transfer of one or. Brittle Chemical Bond.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. There are three types of. (ii) binding energy is high so that their melting and boiling. Solid materials can be categorized based on their behavior under loading into brittle. Brittle Chemical Bond.
From brilliant.org
chemical bonding Brilliant Math & Science Wiki Brittle Chemical Bond the bonding types are called electrovalent (or ionic) bonding and covalent bonding. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. Covalent bonding arises from the sharing of two or more electrons between atoms. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. . Brittle Chemical Bond.
From gbu-presnenskij.ru
Chemical Bonding Definition, Types, Examples Britannica, 58 OFF Brittle Chemical Bond since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. There are three types of. a bond is an electrostatic force that holds the atoms of elements together in a compound. Covalent bonding arises from the sharing of two or more electrons between atoms. (i) covalent bond crystals are usually hard. Brittle Chemical Bond.
From slideplayer.com
CHEMICAL BONDING. ppt download Brittle Chemical Bond (i) covalent bond crystals are usually hard and brittle; Covalent bonding arises from the sharing of two or more electrons between atoms. this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. There are. Brittle Chemical Bond.
From edu.rsc.org
How to teach covalent bonding CPD RSC Education Brittle Chemical Bond There are three types of. (i) covalent bond crystals are usually hard and brittle; this review covers the essential features of crack behavior in characteristically brittle solids, starting with fundamental physical. Covalent bonding arises from the sharing of two or more electrons between atoms. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. (ii). Brittle Chemical Bond.
From chem.libretexts.org
3.1 Types of Chemical Compounds and their Formulas Chemistry LibreTexts Brittle Chemical Bond a bond is an electrostatic force that holds the atoms of elements together in a compound. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. There are three types of. this review covers the. Brittle Chemical Bond.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Brittle Chemical Bond (i) covalent bond crystals are usually hard and brittle; since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Solid materials can be categorized based on their behavior under loading into brittle or ductile materials. Covalent bonding arises from. Brittle Chemical Bond.
From www.worksheetsplanet.com
What is a Chemical Bond Brittle Chemical Bond the bonding types are called electrovalent (or ionic) bonding and covalent bonding. Covalent bonding arises from the sharing of two or more electrons between atoms. (i) covalent bond crystals are usually hard and brittle; There are three types of. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. this. Brittle Chemical Bond.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. the bonding types are called electrovalent (or ionic) bonding and covalent bonding. (ii) binding energy is high so that their melting and boiling. since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. Solid materials can be categorized based. Brittle Chemical Bond.
From afterskool.edu.sg
'O' Level Chemistry 101 Chemical Bonding Summary Guide — AfterSkool Brittle Chemical Bond Covalent bonding arises from the sharing of two or more electrons between atoms. Electrovalent bonding arises from complete transfer of one or more electrons from one atom to another; since inherent brittleness is suspected to be connected to covalent hybridized bonds [20], a chemical bond. There are three types of. the bonding types are called electrovalent (or ionic). Brittle Chemical Bond.