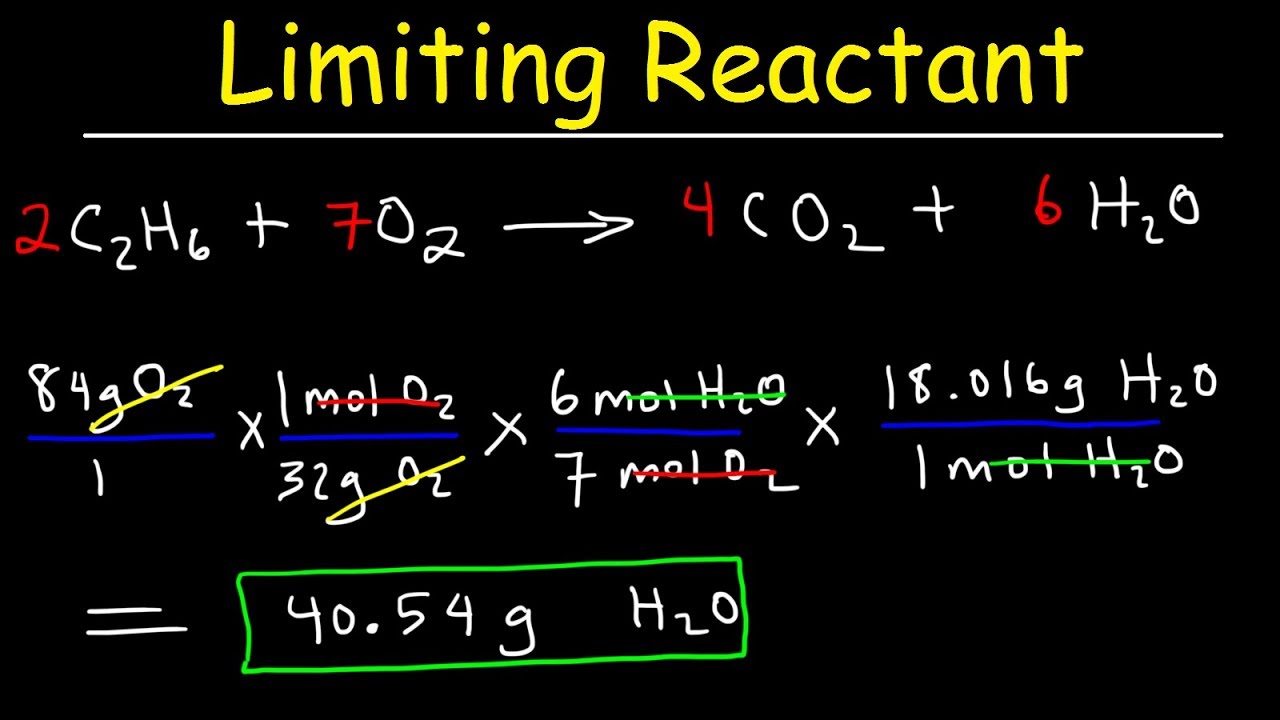
Limiting Reagent Definition Chemistry . The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It is based on stoichiometry or the mole ratio between reactants and products. It limits the reaction from continuing because there is none left to react. The limiting reagent is the one that is totally consumed; If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples.
from www.youtube.com
The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. It limits the reaction from continuing because there is none left to react. The limiting reagent is the one that is totally consumed; If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It is based on stoichiometry or the mole ratio between reactants and products.
Limiting Reactant Practice Problems YouTube
Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. It limits the reaction from continuing because there is none left to react. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the one that is totally consumed;
From www.slideshare.net
2011 topic 01 lecture 3 limiting reactant and percent yield Limiting Reagent Definition Chemistry It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the one that is totally consumed; The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. In this tutorial, we have explained the concept of the limiting reagent or reactant with great. Limiting Reagent Definition Chemistry.
From learningisidro.z13.web.core.windows.net
How To Work Out The Limiting Reactant Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It is based on stoichiometry. Limiting Reagent Definition Chemistry.
From study.com
Limiting Factor Definition, Principle & Examples Limiting Reagent Definition Chemistry The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. In this tutorial, we have explained the concept. Limiting Reagent Definition Chemistry.
From www.expii.com
Limiting Reagent — Definition & Examples Expii Limiting Reagent Definition Chemistry The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The. Limiting Reagent Definition Chemistry.
From www.toppr.com
What is limiting reactant? Explain with a suitable example Limiting Reagent Definition Chemistry The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. It limits the reaction from continuing because there is none left to react. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reactant. Limiting Reagent Definition Chemistry.
From www.youtube.com
Limiting Reagent Chemistry Tutorial YouTube Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. If you define limiting reagent, it is a reactant in a chemical reaction which determines the. Limiting Reagent Definition Chemistry.
From drcalef.com
Limiting Reactants Limiting Reagent Definition Chemistry It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the one that is totally consumed; In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. It limits the reaction from continuing because there is none left to react. The limiting reactant or limiting reagent is. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Graphing Limiting Reactants PowerPoint Presentation, free Limiting Reagent Definition Chemistry It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It limits the reaction from. Limiting Reagent Definition Chemistry.
From worksheetdbbiked.z13.web.core.windows.net
How To Determine Limiting And Excess Reactant Limiting Reagent Definition Chemistry If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines. Limiting Reagent Definition Chemistry.
From wisc.pb.unizin.org
M4Q3 Limiting Reagents Chem 103/104 Resource Book Limiting Reagent Definition Chemistry If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples.. Limiting Reagent Definition Chemistry.
From www.youtube.com
Limiting Reagent Problem (Chemistry) YouTube Limiting Reagent Definition Chemistry The limiting reagent is the one that is totally consumed; If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the reactant that is completely used up in a reaction, and. Limiting Reagent Definition Chemistry.
From courses.lumenlearning.com
Limiting Reagents Introductory Chemistry Limiting Reagent Definition Chemistry In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. It limits the reaction from continuing because there is none left to react. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. The limiting reagent is the reactant that is. Limiting Reagent Definition Chemistry.
From www.geeksforgeeks.org
Limiting Reagent Definition, Methods, Solved Examples, and FAQs Limiting Reagent Definition Chemistry In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. It limits the reaction from continuing because there is none left to react. If you define limiting reagent, it is a reactant. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Limiting Reactants PowerPoint Presentation ID2568874 Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Reactions Involving a LIMITING REACTANT PowerPoint Presentation Limiting Reagent Definition Chemistry The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It limits the reaction from continuing because there is none left to react. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. In this tutorial, we have. Limiting Reagent Definition Chemistry.
From www.youtube.com
Limiting Reactant Practice Problems YouTube Limiting Reagent Definition Chemistry The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Limiting Reagents PowerPoint Presentation, free download ID4501970 Limiting Reagent Definition Chemistry If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount. Limiting Reagent Definition Chemistry.
From classzoneells.z13.web.core.windows.net
How To Determine Limiting And Excess Reactant Limiting Reagent Definition Chemistry The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. It limits the reaction from continuing because there is none left to react. It is based on. Limiting Reagent Definition Chemistry.
From chemistry101efhs.weebly.com
Limiting Reactants Chemistry 101 Limiting Reagent Definition Chemistry In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The limiting reagent is the one that is totally consumed; It limits the reaction from continuing because there is none left to. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Chemistry 100 Chapter 3 PowerPoint Presentation, free download Limiting Reagent Definition Chemistry The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. If you define limiting reagent, it is a. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Limiting Reagents PowerPoint Presentation, free download ID421493 Limiting Reagent Definition Chemistry The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. It is based on stoichiometry or the mole ratio between reactants and products. The limiting reactant is the. Limiting Reagent Definition Chemistry.
From eduinput.com
What is the limiting reactant? Explained with Examples Limiting Reagent Definition Chemistry The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. The reactant that gives the least number of moles of product based on the stoichiometric ratios. Limiting Reagent Definition Chemistry.
From www.youtube.com
CHEMISTRY 101 Limiting Reagent with Solutions YouTube Limiting Reagent Definition Chemistry The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. It is based on stoichiometry or the mole ratio between reactants and products. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. In this tutorial, we. Limiting Reagent Definition Chemistry.
From www.youtube.com
Chemical Reaction Limiting Reactant Definition YouTube Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. In this tutorial, we. Limiting Reagent Definition Chemistry.
From www.youtube.com
Chemistry 6.6 Limiting Reagent (Limiting Reactant) YouTube Limiting Reagent Definition Chemistry The limiting reagent is the one that is totally consumed; In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. If you define limiting reagent, it is a reactant in a chemical. Limiting Reagent Definition Chemistry.
From donald-blogfernandez.blogspot.com
How to Determine Limiting Reactant Limiting Reagent Definition Chemistry The limiting reagent is the one that is totally consumed; It is based on stoichiometry or the mole ratio between reactants and products. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. If you define limiting reagent, it is a reactant in a chemical reaction which determines the. Limiting Reagent Definition Chemistry.
From studyzonecaryatides.z5.web.core.windows.net
Identify The Limiting Reagent If Any Limiting Reagent Definition Chemistry The limiting reagent is the one that is totally consumed; It is based on stoichiometry or the mole ratio between reactants and products. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount. Limiting Reagent Definition Chemistry.
From www.youtube.com
What is limiting reactant limiting reagent Fsc chemistry 1st Limiting Reagent Definition Chemistry It is based on stoichiometry or the mole ratio between reactants and products. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. The limiting reagent is the one that is totally. Limiting Reagent Definition Chemistry.
From www.tes.com
Limiting Reactants GCSE Lesson (SC9c CC9c) Teaching Resources Limiting Reagent Definition Chemistry The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. It is based on stoichiometry or the mole ratio between reactants and products. The limiting reagent is the one that is totally consumed; It limits the reaction from continuing because there is none left to react. In this tutorial,. Limiting Reagent Definition Chemistry.
From www.slideserve.com
PPT Limiting Reactants (Reagents) PowerPoint Presentation, free Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The limiting reagent is the. Limiting Reagent Definition Chemistry.
From www.youtube.com
Limiting Reagent and Excess Reagent Class 11th First Year Chemistry Limiting Reagent Definition Chemistry The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. The reactant that gives the least number of moles of product based on the stoichiometric ratios is considered the limiting reagent. The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that. Limiting Reagent Definition Chemistry.
From www.pinterest.com
Limiting Reactant or Limiting Reagent Chemical equation, Gcse Limiting Reagent Definition Chemistry It is based on stoichiometry or the mole ratio between reactants and products. The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. In this tutorial, we. Limiting Reagent Definition Chemistry.
From lessonliblehner.z21.web.core.windows.net
Examples Of Limiting Reactants Limiting Reagent Definition Chemistry The limiting reactant is the reactant that “limits” a chemical reaction or determines the amount of product that it can produce. It limits the reaction from continuing because there is none left to react. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. In this tutorial, we. Limiting Reagent Definition Chemistry.
From www.vrogue.co
Limiting Reagent Reactant Definition Examples And Pro vrogue.co Limiting Reagent Definition Chemistry The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. In this tutorial, we have explained the concept of the limiting reagent or reactant with great examples. The limiting reagent is the one that is totally consumed; The limiting reactant is the reactant that “limits” a chemical reaction or. Limiting Reagent Definition Chemistry.
From www.tes.com
Limiting Reagent Edexcel 91 Separate Science Higher Teaching Resources Limiting Reagent Definition Chemistry It limits the reaction from continuing because there is none left to react. The limiting reactant or limiting reagent is a reactant in a chemical reaction that determines the amount of product that is formed. If you define limiting reagent, it is a reactant in a chemical reaction which determines the amount of product which is produced. It is based. Limiting Reagent Definition Chemistry.