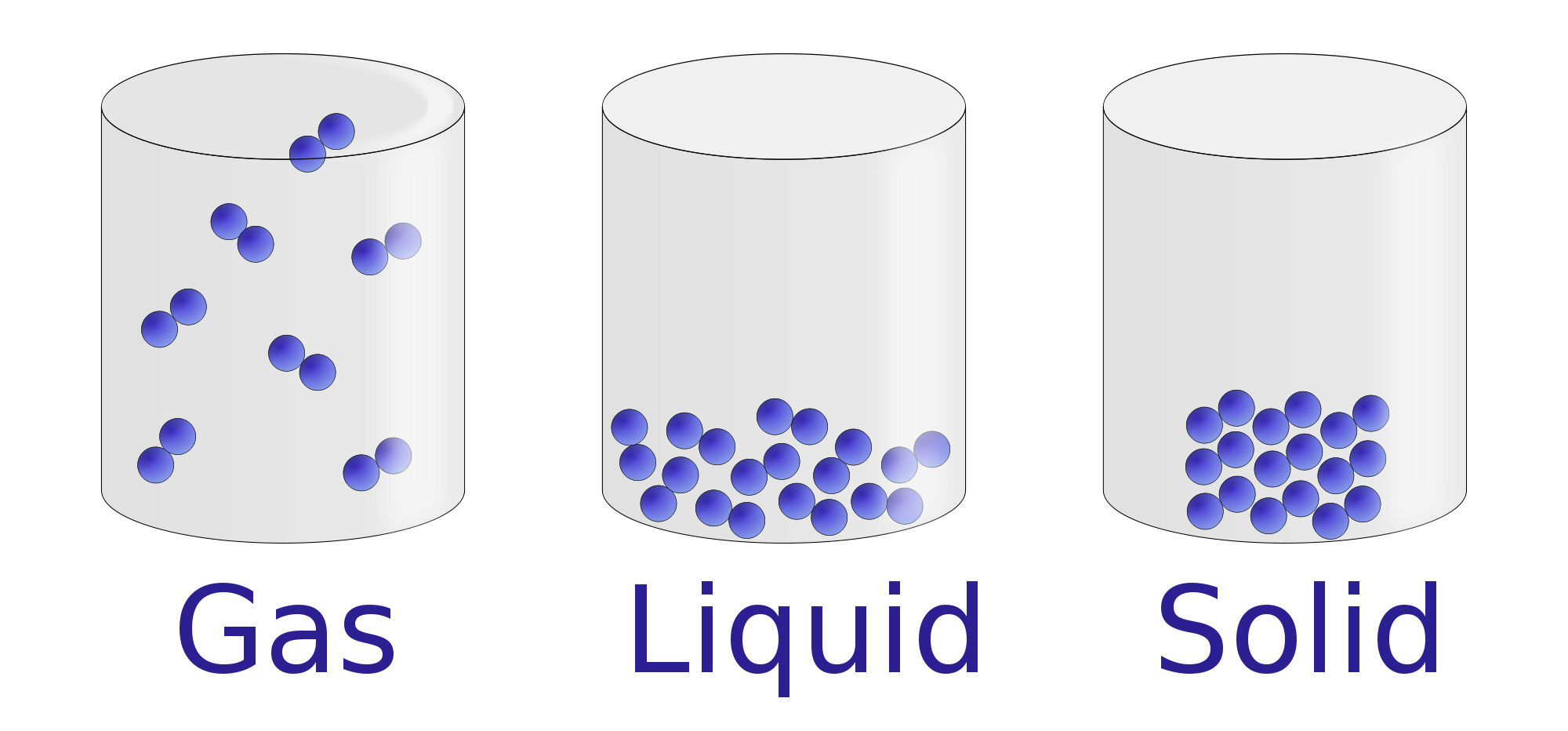
Solid To Liquid Chemistry . Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The conversion of a solid to a liquid is called fusion (or melting). Watch different types of molecules form a solid, liquid, or gas. Solid, liquid, or gas (1). Add or remove heat and watch the phase change. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Each substance has three phases it can change into; Change the temperature or volume of a container and see a pressure. The conversion of a solid to a liquid is called fusion (or melting).
from www.visionlearning.com
The conversion of a solid to a liquid is called fusion (or melting). Add or remove heat and watch the phase change. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The conversion of a solid to a liquid is called fusion (or melting). Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Each substance has three phases it can change into; Solid, liquid, or gas (1). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Change the temperature or volume of a container and see a pressure.
Properties of Liquids Chemistry Visionlearning
Solid To Liquid Chemistry Add or remove heat and watch the phase change. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Solid, liquid, or gas (1). The conversion of a solid to a liquid is called fusion (or melting). The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Watch different types of molecules form a solid, liquid, or gas. Add or remove heat and watch the phase change. Each substance has three phases it can change into; Change the temperature or volume of a container and see a pressure. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure.
From www.youtube.com
States of Matter Solid, Liquid and Gas Chemistry YouTube Solid To Liquid Chemistry The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The. Solid To Liquid Chemistry.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Chemistry Watch different types of molecules form a solid, liquid, or gas. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The conversion of a solid to a liquid is called fusion (or melting). Add or remove heat and watch the phase change. Phase transition is. Solid To Liquid Chemistry.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid To Liquid Chemistry Add or remove heat and watch the phase change. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called. Solid To Liquid Chemistry.
From www.britannica.com
solid Definition & Facts Britannica Solid To Liquid Chemistry Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Watch different types of molecules form a solid, liquid, or gas. Each substance has three phases it can change into; Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. Solid To Liquid Chemistry.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Each substance has three phases it can change into; The conversion of a solid to a liquid is called fusion (or melting). Every. Solid To Liquid Chemistry.
From www.alamy.com
Different states of matter solid, liquid, gas vector diagram. Set of Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Solid, liquid, or gas (1). Change the temperature or volume of a container and see a pressure. The energy required to melt 1 mol of a substance is its. Solid To Liquid Chemistry.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Change the temperature or volume of a container and see a pressure. Add or remove heat and watch the phase change. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Watch different types of molecules form a solid,. Solid To Liquid Chemistry.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To Liquid Chemistry Change the temperature or volume of a container and see a pressure. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Each substance has three phases it can change into; Solid, liquid, or gas (1). The conversion of a solid to a liquid is called fusion (or melting). Add or remove heat. Solid To Liquid Chemistry.
From manuallistcantabank.z21.web.core.windows.net
Solid Liquid And Gas Diagram Solid To Liquid Chemistry Change the temperature or volume of a container and see a pressure. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy required to melt 1 mol of a substance is its enthalpy. Solid To Liquid Chemistry.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Chemistry Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Every element and substance can transition from one phase to another at a specific combination of temperature and. Solid To Liquid Chemistry.
From www.alamy.com
matter in different states for example water. solid, liquid, gas and Solid To Liquid Chemistry Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Add or remove heat and watch the phase change. The conversion of a solid to a liquid is called fusion (or melting). Change the temperature or volume of a container and see a pressure. Every element and substance can transition from one. Solid To Liquid Chemistry.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Solid To Liquid Chemistry Each substance has three phases it can change into; Solid, liquid, or gas (1). The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). Every element and. Solid To Liquid Chemistry.
From chem.libretexts.org
13.2 Saturated Solutions and Solubility Chemistry LibreTexts Solid To Liquid Chemistry Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Change the temperature or volume of a container and see a pressure. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or. Solid To Liquid Chemistry.
From courses.lumenlearning.com
Phases and Classification of Matter Chemistry Solid To Liquid Chemistry The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solid, liquid, or gas (1). The conversion of a solid to a liquid is called fusion (or melting). Each substance has three phases it can change into; Phase transition is when a substance changes from a solid, liquid, or gas state to a. Solid To Liquid Chemistry.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid To Liquid Chemistry Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Add or remove heat and watch the phase change. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Change the temperature or volume of a container and see. Solid To Liquid Chemistry.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid To Liquid Chemistry Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus).. Solid To Liquid Chemistry.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion Solid To Liquid Chemistry Each substance has three phases it can change into; Solid, liquid, or gas (1). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Under some circumstances, the solid phase can transition directly to the. Solid To Liquid Chemistry.
From www.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Each substance has three phases it can change into; Add or remove heat and watch the phase change. Change the temperature or volume. Solid To Liquid Chemistry.
From saylordotorg.github.io
Solids and Liquids Solid To Liquid Chemistry Each substance has three phases it can change into; The conversion of a solid to a liquid is called fusion (or melting). Add or remove heat and watch the phase change. The conversion of a solid to a liquid is called fusion (or melting). Every element and substance can transition from one phase to another at a specific combination of. Solid To Liquid Chemistry.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations Solid To Liquid Chemistry Each substance has three phases it can change into; The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Under some circumstances, the solid phase can transition directly to the gas phase without going through. Solid To Liquid Chemistry.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Solid To Liquid Chemistry Each substance has three phases it can change into; Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The conversion of a solid to a liquid is called fusion (or melting). Watch different types of molecules form a solid, liquid, or gas. Phase transition is. Solid To Liquid Chemistry.
From www.youtube.com
States of Matter Solids, Liquids, Gases & Plasma Chemistry YouTube Solid To Liquid Chemistry Each substance has three phases it can change into; Solid, liquid, or gas (1). Add or remove heat and watch the phase change. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1. Solid To Liquid Chemistry.
From sciencenotes.org
States of Matter Solid To Liquid Chemistry The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). Add or remove heat and watch the phase change. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transition. Solid To Liquid Chemistry.
From www.teachoo.com
Interconversion of States of Matter with Flow Chart Teachoo Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Each substance has three phases it can change into; Change the temperature or volume of a container and see a pressure. Add or remove heat and watch the phase change.. Solid To Liquid Chemistry.
From www.ase.org.uk
Solids, liquids and gases Solid To Liquid Chemistry Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Each substance has three phases it can change into; Watch different types of molecules form a solid, liquid, or gas. The conversion of a solid to a liquid is called fusion (or melting). The energy required. Solid To Liquid Chemistry.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff Solid To Liquid Chemistry Change the temperature or volume of a container and see a pressure. Watch different types of molecules form a solid, liquid, or gas. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid. Solid To Liquid Chemistry.
From saylordotorg.github.io
Solids and Liquids Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Each substance has three phases it can change into; The conversion of a solid to a liquid is called fusion (or melting). Every element and substance can transition from. Solid To Liquid Chemistry.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid To Liquid Chemistry Add or remove heat and watch the phase change. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Change the temperature or volume of a container and see a pressure. Watch different. Solid To Liquid Chemistry.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry Solid To Liquid Chemistry Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The conversion of a solid to a liquid is called fusion (or melting). Watch different types of molecules form a solid, liquid, or gas. Solid, liquid, or gas (1). The conversion of a solid to a liquid is called fusion (or melting).. Solid To Liquid Chemistry.
From schoolbag.info
For a given substance, do you expect the density of the substance in Solid To Liquid Chemistry Add or remove heat and watch the phase change. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Watch different types of molecules form a solid, liquid, or gas. Solid, liquid, or gas (1). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state.. Solid To Liquid Chemistry.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Solid To Liquid Chemistry The conversion of a solid to a liquid is called fusion (or melting). Watch different types of molecules form a solid, liquid, or gas. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Solid, liquid, or gas (1). Change the temperature or volume of a container and see a pressure.. Solid To Liquid Chemistry.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solid To Liquid Chemistry Watch different types of molecules form a solid, liquid, or gas. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Solid, liquid, or gas (1). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Every element. Solid To Liquid Chemistry.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid To Liquid Chemistry Add or remove heat and watch the phase change. Solid, liquid, or gas (1). Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. The conversion of. Solid To Liquid Chemistry.
From learningschoolboyer.z13.web.core.windows.net
Show The Phase Change Diagram Process Solid To Liquid Chemistry Solid, liquid, or gas (1). Each substance has three phases it can change into; Watch different types of molecules form a solid, liquid, or gas. Change the temperature or volume of a container and see a pressure. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Under some circumstances, the. Solid To Liquid Chemistry.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Solid To Liquid Chemistry Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Solid, liquid, or gas (1). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Add or remove heat and watch the phase change. Under some circumstances, the solid phase can transition directly to the. Solid To Liquid Chemistry.