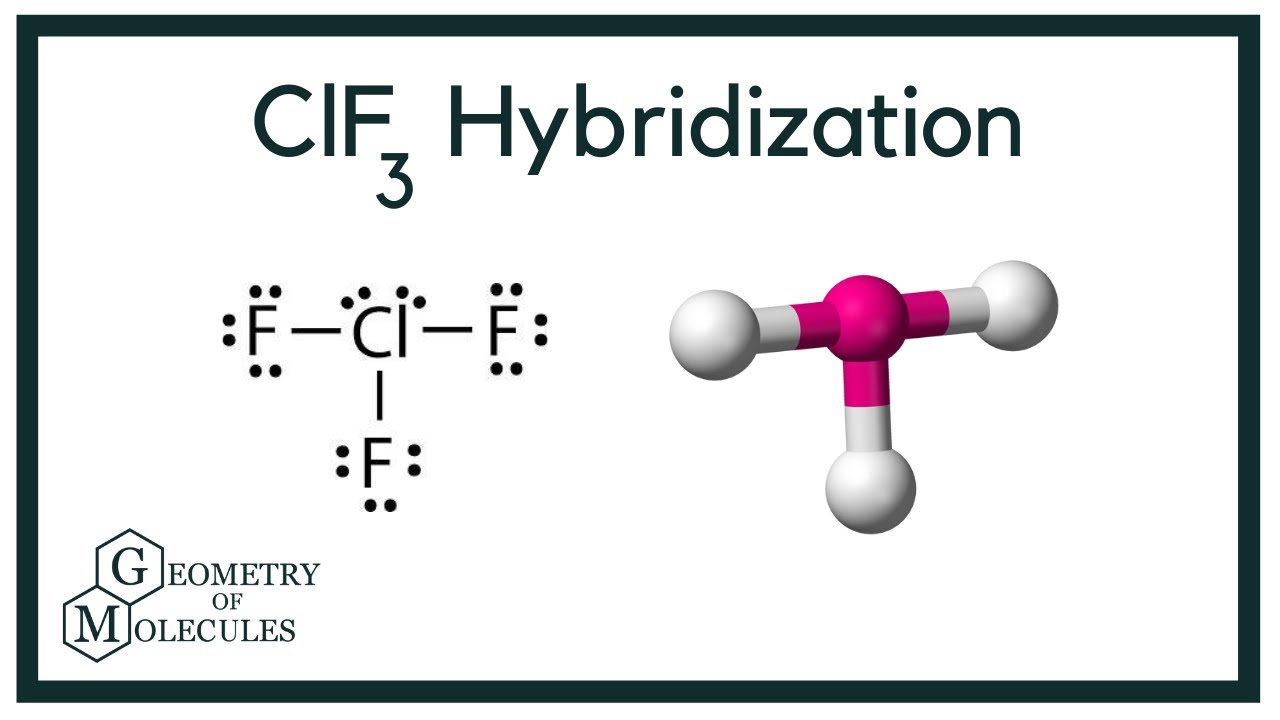
Chlorine Trifluoride Oxidation States . Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Its chemical formula is clf 3. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine trifluoride is a chemical compound. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Syntheses and characterization of difluorooxychloronium(v). The chlorine is in its +3 oxidation state. It contains chlorine and fluoride ions.
from www.youtube.com
Chlorine trifluoride is a chemical compound. It contains chlorine and fluoride ions. The chlorine is in its +3 oxidation state. Syntheses and characterization of difluorooxychloronium(v). Chlorine and fluorine are nasty enough by themselves, but at least in. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Its chemical formula is clf 3.
ClF3 Hybridization (Chlorine Trifluoride) YouTube
Chlorine Trifluoride Oxidation States Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Its chemical formula is clf 3. It contains chlorine and fluoride ions. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. The chlorine is in its +3 oxidation state. Chlorine and fluorine are nasty enough by themselves, but at least in. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine trifluoride is a chemical compound. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Oxidation States Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine trifluoride (clf 3) is. Chlorine Trifluoride Oxidation States.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Chlorine Trifluoride Oxidation States Syntheses and characterization of difluorooxychloronium(v). Chlorine and fluorine are nasty enough by themselves, but at least in. Its chemical formula is clf 3. Chlorine trifluoride is a chemical compound. It contains chlorine and fluoride ions. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. The reactions of chlorine trifluoride with. Chlorine Trifluoride Oxidation States.
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Chlorine Trifluoride Oxidation States Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine and fluorine are nasty enough by themselves, but at least in. Its chemical formula is clf 3. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine forms a. Chlorine Trifluoride Oxidation States.
From www.toppr.com
Chlorine atom in its third excited state with fluorine to form a Chlorine Trifluoride Oxidation States The chlorine is in its +3 oxidation state. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride is a chemical compound. Chlorine trifluoride (clf 3) is a compound of significant interest due to its. Chlorine Trifluoride Oxidation States.
From www.chemeurope.com
Periodic table of oxidations states Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride is a chemical compound. The chlorine is in its +3 oxidation state. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride. Chlorine Trifluoride Oxidation States.
From www.youtube.com
Oxides of ChlorineCh5The Halogens and the Noble gasesLec6b YouTube Chlorine Trifluoride Oxidation States Chlorine trifluoride is a chemical compound. It contains chlorine and fluoride ions. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. The reactions of chlorine trifluoride with oxides or. Chlorine Trifluoride Oxidation States.
From readingandwritingprojectcom.web.fc2.com
what is the electronic geometry of clf3 Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine and fluorine are nasty enough by themselves, but at least in. It contains chlorine and fluoride ions. Its chemical formula. Chlorine Trifluoride Oxidation States.
From www.wikiwand.com
Chlorine trifluoride oxide Wikiwand Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Syntheses and characterization of difluorooxychloronium(v). The chlorine is in its +3 oxidation state. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine and fluorine are nasty enough by themselves, but at least in. The reactions of chlorine trifluoride with oxides or. Chlorine Trifluoride Oxidation States.
From www.youtube.com
ClF3 Hybridization (Chlorine Trifluoride) YouTube Chlorine Trifluoride Oxidation States The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride is a chemical compound. Its chemical formula is clf 3. Chlorine trifluoride (which i will subsequently refer to as. Chlorine Trifluoride Oxidation States.
From www.slideserve.com
PPT Valency & bonding, Oxidation States and redox reaction PowerPoint Chlorine Trifluoride Oxidation States Its chemical formula is clf 3. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine trifluoride is a chemical compound. Syntheses and characterization of difluorooxychloronium(v). It contains chlorine and fluoride ions. Chlorine trifluoride (clf. Chlorine Trifluoride Oxidation States.
From onlinelibrary.wiley.com
Photochemistry with ClF3 An Access to [ClOF2]+ Salts Scheibe 2022 Chlorine Trifluoride Oxidation States Chlorine trifluoride is a chemical compound. Chlorine and fluorine are nasty enough by themselves, but at least in. It contains chlorine and fluoride ions. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Its chemical. Chlorine Trifluoride Oxidation States.
From www.numerade.com
SOLVED Gaseous chlorine trifluoride is a compound used in the Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. It contains chlorine and fluoride ions. Chlorine trifluoride (clf 3) is a compound of significant interest due to its. Chlorine Trifluoride Oxidation States.
From www.chegg.com
Solved 1. Chlorine oxidation states. Chlorine exists in 7 Chlorine Trifluoride Oxidation States Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Its chemical formula is clf 3. Chlorine trifluoride is a chemical compound. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride (which. Chlorine Trifluoride Oxidation States.
From www.youtube.com
How to find the Oxidation Number for Cl in ClF3 (Chlorine trifluoride Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Chlorine trifluoride is a chemical compound. The chlorine is in its +3 oxidation state. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine and fluorine are nasty enough. Chlorine Trifluoride Oxidation States.
From sciencenotes.org
Downloadable Periodic Table Oxidation States Chlorine Trifluoride Oxidation States The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. It contains chlorine and fluoride. Chlorine Trifluoride Oxidation States.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine trifluoride is a chemical compound. Chlorine forms a series of oxides (table \ (\pageindex. Chlorine Trifluoride Oxidation States.
From chem.unc.edu
Chloride Oxidation by One or TwoPhoton Excitation of N Chlorine Trifluoride Oxidation States The chlorine is in its +3 oxidation state. Chlorine trifluoride is a chemical compound. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Syntheses and characterization of difluorooxychloronium(v). It contains chlorine and fluoride ions. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv. Chlorine Trifluoride Oxidation States.
From www.numerade.com
SOLVED Determine the oxidation state for each of the elements below Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv. Chlorine Trifluoride Oxidation States.
From www.onlinechemistrytutor.net
An introduction to oxidation state Online Chemistry Tutor Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. The chlorine is in its +3 oxidation state. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. It. Chlorine Trifluoride Oxidation States.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Trifluoride Oxidation States Chlorine and fluorine are nasty enough by themselves, but at least in. It contains chlorine and fluoride ions. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Its chemical formula is clf 3. The reactions of chlorine trifluoride with oxides or with metals and. Chlorine Trifluoride Oxidation States.
From www.jobilize.com
Fluorides of chlorine, Compounds of chlorine, By OpenStax (Page 2/2 Chlorine Trifluoride Oxidation States Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. It contains chlorine and fluoride ions. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv. Chlorine Trifluoride Oxidation States.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Oxidation States Syntheses and characterization of difluorooxychloronium(v). Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine trifluoride is a chemical compound. It contains chlorine and fluoride ions. Its chemical formula is clf 3. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation. Chlorine Trifluoride Oxidation States.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Oxidation States Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride is a chemical compound. The reactions of chlorine trifluoride with oxides or. Chlorine Trifluoride Oxidation States.
From mavink.com
Periodic Table Of Oxidation States Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride is a chemical compound. Syntheses and characterization of difluorooxychloronium(v). Its chemical formula is clf 3. Chlorine and fluorine are nasty enough by themselves, but at least in. It contains chlorine and fluoride ions. Chlorine forms a series of. Chlorine Trifluoride Oxidation States.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Oxidation States It contains chlorine and fluoride ions. Chlorine trifluoride is a chemical compound. Its chemical formula is clf 3. The chlorine is in its +3 oxidation state. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride (clf 3) is a compound of. Chlorine Trifluoride Oxidation States.
From www.22measured.com
SOLVED The reaction of hydrazine (N2H4) and chlorine trifluoride (ClF3 Chlorine Trifluoride Oxidation States Chlorine trifluoride is a chemical compound. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. The. Chlorine Trifluoride Oxidation States.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃ Chlorine Trifluoride Oxidation States Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. The chlorine is in its +3 oxidation state. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. The. Chlorine Trifluoride Oxidation States.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Oxidation States Syntheses and characterization of difluorooxychloronium(v). The chlorine is in its +3 oxidation state. Its chemical formula is clf 3. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine and fluorine are nasty enough by themselves, but at least in. The reactions of chlorine trifluoride with oxides or. Chlorine Trifluoride Oxidation States.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Trifluoride Oxidation States Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex. Chlorine Trifluoride Oxidation States.
From askfilo.com
Oxidation states of chlorine in NOCl and OCl2 are Filo Chlorine Trifluoride Oxidation States Chlorine trifluoride is a chemical compound. Chlorine and fluorine are nasty enough by themselves, but at least in. It contains chlorine and fluoride ions. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive. Chlorine Trifluoride Oxidation States.
From www.researchgate.net
(PDF) Reactions of Chlorine Dioxide with Organic Compounds Chlorine Trifluoride Oxidation States Chlorine trifluoride is a chemical compound. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation of the compounds [clof 2][mf 6] (m=v, nb, ta, ru, os, ir, p, sb). Its chemical formula is clf 3. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine. Chlorine Trifluoride Oxidation States.
From linatusims.blogspot.com
Oxidation Number of Chlorine LinatuSims Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Its chemical formula is clf 3. Syntheses and characterization of difluorooxychloronium(v). Chlorine trifluoride is a chemical compound. The chlorine. Chlorine Trifluoride Oxidation States.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Chlorine Trifluoride Oxidation States Its chemical formula is clf 3. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. Chlorine and fluorine are nasty enough by themselves, but at least in. Syntheses and. Chlorine Trifluoride Oxidation States.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Chlorine Trifluoride Oxidation States Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,.. Chlorine Trifluoride Oxidation States.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Trifluoride Oxidation States Chlorine trifluoride (clf 3) is a compound of significant interest due to its strong oxidizing properties and diverse industrial. Chlorine forms a series of oxides (table \ (\pageindex {2}\)) in which the chlorine has the formal oxidation states +1, +4,. The reactions of chlorine trifluoride with oxides or with metals and o 2 under uv irradiation led to the formation. Chlorine Trifluoride Oxidation States.