Iron How Many Valence Electrons . 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Find out how iron forms bonds, why it has two valencies and how to write its configuration. There are two electrons in the 4s subshell and 6. Learn the definition and examples of valence electrons. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of valence electrons for iron is 8: Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell.
from mavink.com
93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of valence electrons for iron is 8: Find out how iron forms bonds, why it has two valencies and how to write its configuration. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Learn the definition and examples of valence electrons. 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6.
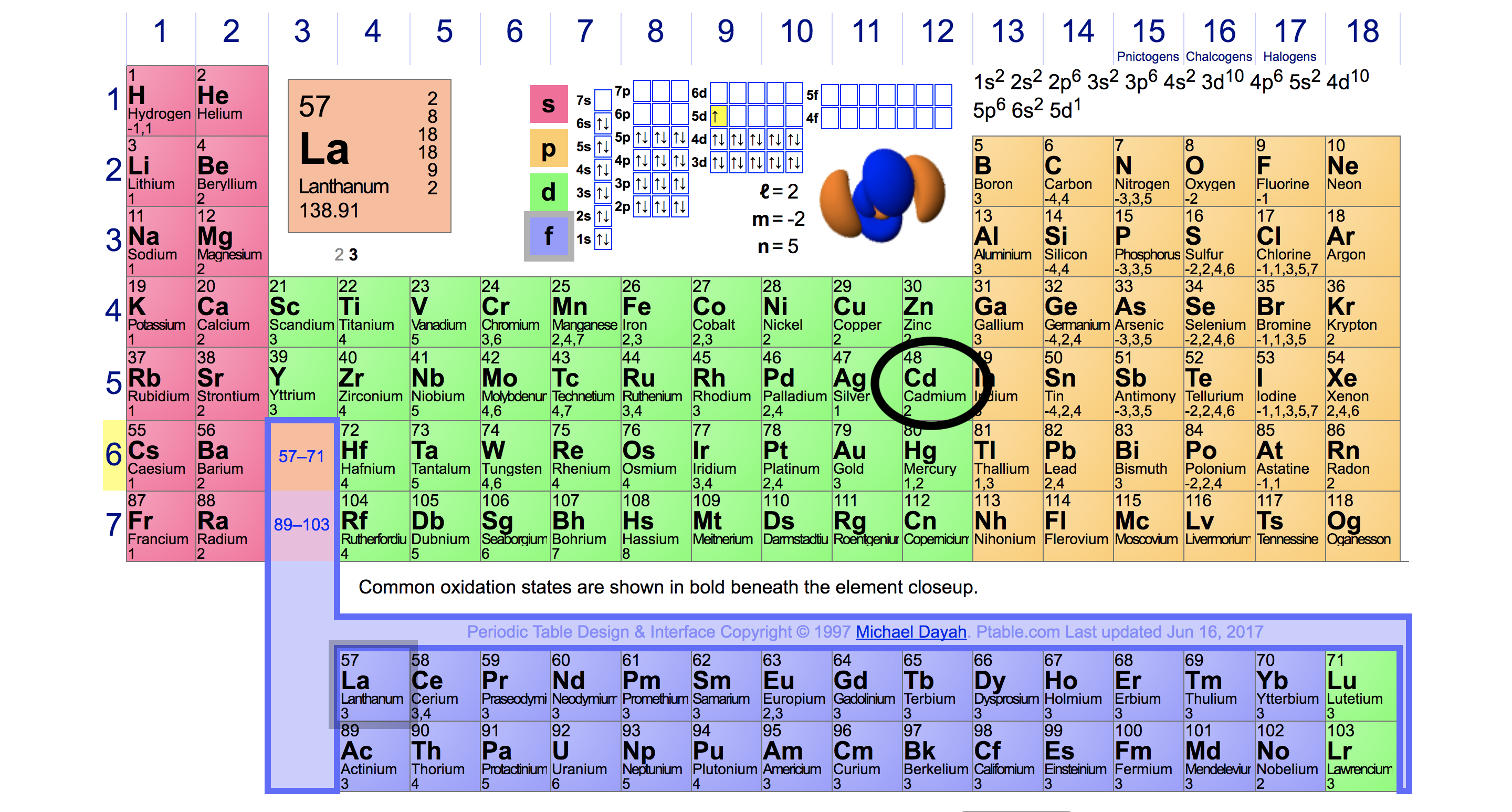
Periodic Table Valence Electrons
Iron How Many Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. The total number of valence electrons for iron is 8: There are two electrons in the 4s subshell and 6. Learn the definition and examples of valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Find out how iron forms bonds, why it has two valencies and how to write its configuration. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron How Many Valence Electrons 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Find out how iron forms bonds, why it has two valencies and how to write. Iron How Many Valence Electrons.
From farmerlextre.blogspot.com
Number Of Electrons In Iron Farmer Lextre Iron How Many Valence Electrons Learn the definition and examples of valence electrons. The total number of valence electrons for iron is 8: There are two electrons in the 4s subshell and 6. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with. Iron How Many Valence Electrons.
From www.youtube.com
Transition Metals Periodic table Chemistry Khan Academy YouTube Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. Find out how iron forms bonds, why it has two valencies and how to write its configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. 119 rows find the valence electrons of any element in the periodic. Iron How Many Valence Electrons.
From topblogtenz.com
How to find Valence electrons Various method and Examples Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, why it has. Iron How Many Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron How Many Valence Electrons Learn the definition and examples of valence electrons. 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Find out how iron forms bonds, why it has two valencies and how to write its configuration. Iron is a transition metal, so the number of valence electrons includes those in the. Iron How Many Valence Electrons.
From alquilercastilloshinchables.info
8 Photos Iron Periodic Table And Description Alqu Blog Iron How Many Valence Electrons 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Find out how iron forms bonds, why it has two valencies and how to write its configuration. Learn the definition and examples of valence electrons. 119 rows find the valence electrons of any element in the periodic table, including. Iron How Many Valence Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron How Many Valence Electrons Find out how iron forms bonds, why it has two valencies and how to write its configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. There are two electrons in the 4s subshell and 6. Learn the definition and examples of valence electrons. The total number of. Iron How Many Valence Electrons.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID5581383 Iron How Many Valence Electrons Learn the definition and examples of valence electrons. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of valence electrons for iron is 8: Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in. Iron How Many Valence Electrons.
From www.youtube.com
How many valence electrons does iron have? YouTube Iron How Many Valence Electrons The total number of valence electrons for iron is 8: Learn the definition and examples of valence electrons. Find out how iron forms bonds, why it has two valencies and how to write its configuration. There are two electrons in the 4s subshell and 6. Iron is a transition metal, so the number of valence electrons includes those in the. Iron How Many Valence Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Iron How Many Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond. Iron How Many Valence Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron How Many Valence Electrons Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, why it has two valencies and how to write its configuration. There are two electrons in the 4s subshell and 6. The total number of valence electrons for iron is 8: Learn the definition and examples of. Iron How Many Valence Electrons.
From mavink.com
Periodic Table Valence Electrons Iron How Many Valence Electrons Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, why it has two valencies and how to write its configuration. Learn the definition and examples of valence electrons. The total number of valence electrons for iron is 8: 93 rows learn the valences of the chemical. Iron How Many Valence Electrons.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. The total number of valence electrons for iron is. Iron How Many Valence Electrons.
From www.chegg.com
Solved ipoint How many valence electrons does iron have? 1 Iron How Many Valence Electrons Learn the definition and examples of valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. There are two electrons in the 4s subshell and 6. The. Iron How Many Valence Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. The total number of valence electrons for iron is 8: Learn the definition and examples of valence electrons. Find out how iron forms bonds, why it has two valencies and how to write its configuration. 119 rows find the valence electrons of any element in the periodic table, including nitrogen. Iron How Many Valence Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Iron How Many Valence Electrons Learn the definition and examples of valence electrons. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6. 119. Iron How Many Valence Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. The total number of valence electrons for iron is 8: 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those. Iron How Many Valence Electrons.
From study.com
Valence Electrons Definition, Role & Examples Video & Lesson Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. Learn the definition and examples of valence electrons. Find out how iron forms bonds, why it has two valencies and how to write its configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of. Iron How Many Valence Electrons.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron How Many Valence Electrons Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6. Learn the definition and examples of valence electrons. The. Iron How Many Valence Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Iron How Many Valence Electrons The total number of valence electrons for iron is 8: 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Find out how iron forms bonds, why it has two valencies and how to write its configuration. 119 rows find the valence electrons of any element in the periodic. Iron How Many Valence Electrons.
From www.clipartmax.com
Diagram, Atom, Iron, Atomic, Bohr, Nucleus Many Valence Electrons Iron How Many Valence Electrons Learn the definition and examples of valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Find out how iron forms bonds, why it has two valencies and how to write its configuration. The total number of valence electrons for iron is 8: 93. Iron How Many Valence Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Iron How Many Valence Electrons 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Learn the. Iron How Many Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Iron How Many Valence Electrons The total number of valence electrons for iron is 8: Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6. Find out how iron forms bonds, why it has two valencies and how to write its. Iron How Many Valence Electrons.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements Iron How Many Valence Electrons There are two electrons in the 4s subshell and 6. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. 93 rows learn the valences of the chemical elements,. Iron How Many Valence Electrons.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron How Many Valence Electrons Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Learn the definition and examples of valence electrons. Iron is a transition metal, so the number of valence electrons includes those in. Iron How Many Valence Electrons.
From ar.inspiredpencil.com
Iron Orbital Notation Iron How Many Valence Electrons Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Find out how iron forms bonds, why it has two valencies and how to write its configuration. There are. Iron How Many Valence Electrons.
From utedzz.blogspot.com
Valence Electrons Periodic Table Transition Metals Periodic Table Iron How Many Valence Electrons 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Find out how iron forms bonds, why it has two valencies and how to write its configuration. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the. Iron How Many Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron How Many Valence Electrons Find out how iron forms bonds, why it has two valencies and how to write its configuration. The total number of valence electrons for iron is 8: Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the definition and examples of valence electrons. Learn. Iron How Many Valence Electrons.
From www.dreamstime.com
Iron Atom Stock Illustrations 1,568 Iron Atom Stock Illustrations Iron How Many Valence Electrons 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. There are two electrons in the 4s subshell and 6. The total number of valence electrons for iron is 8: Find out how iron forms bonds, why it has two valencies and how to write its configuration. Learn the. Iron How Many Valence Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron How Many Valence Electrons The total number of valence electrons for iron is 8: Learn the definition and examples of valence electrons. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in. Iron How Many Valence Electrons.
From periodictable.me
How Many Valence Electrons Does Iron have Archives Dynamic Periodic Iron How Many Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of valence. Iron How Many Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron How Many Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. The total number of valence electrons for iron is 8: Learn the definition and examples of valence electrons. 93. Iron How Many Valence Electrons.
From ar.inspiredpencil.com
Periodic Table Valence Electrons Iron How Many Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d subshell, not just those in the 4s subshell. There are two electrons in the 4s subshell and 6. 119 rows find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Find out how iron forms bonds,. Iron How Many Valence Electrons.
From www.youtube.com
How to Determine Number of Valence Electrons in Element, Ion, and Iron How Many Valence Electrons 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The total number of valence electrons for iron is 8: Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. Learn the definition and examples of valence electrons. Iron is a transition. Iron How Many Valence Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron How Many Valence Electrons Find out how iron forms bonds, why it has two valencies and how to write its configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Learn the electronic configuration of iron (fe) with 26 electrons, its oxidation states, allotropic forms and applications. There are two electrons in. Iron How Many Valence Electrons.