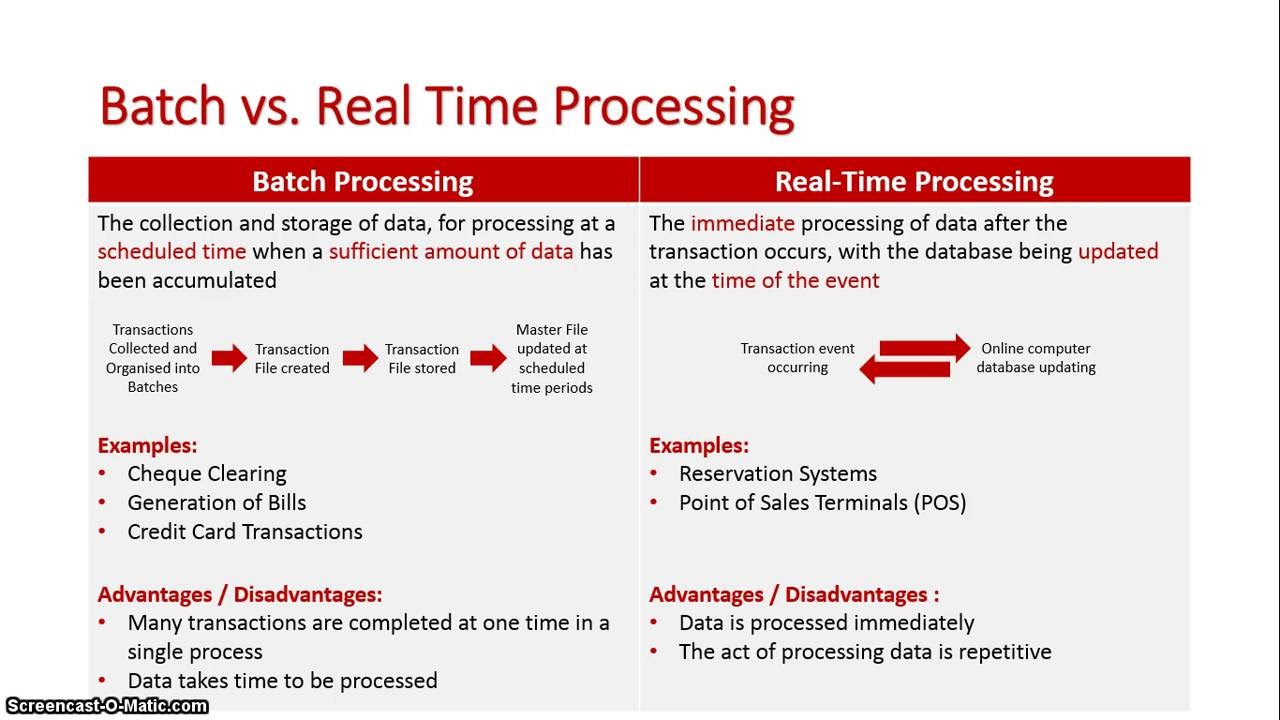
Medical Device Batch Definition . 211.188 batch production and control records. batch (or lot): Batch production and control records shall be prepared for each. A specific quantity of material produced in a process or series of processes so that it is expected. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. the drug constituent of a combination (drug and medical device) product. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. These documents are typically used and completed by the.
from mavink.com
(a) to assure uniformity from batch to batch, master production and control records for each drug product, including. the drug constituent of a combination (drug and medical device) product. 211.188 batch production and control records. A specific quantity of material produced in a process or series of processes so that it is expected. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; batch (or lot): Batch production and control records shall be prepared for each. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. These documents are typically used and completed by the.
Examples Of Batch Processing
Medical Device Batch Definition These documents are typically used and completed by the. batch (or lot): the drug constituent of a combination (drug and medical device) product. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. These documents are typically used and completed by the. 211.188 batch production and control records. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. A specific quantity of material produced in a process or series of processes so that it is expected. Batch production and control records shall be prepared for each. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition;
From dxochszdk.blob.core.windows.net
Medical Equipment Validation at Betty Sanchez blog Medical Device Batch Definition the drug constituent of a combination (drug and medical device) product. 211.188 batch production and control records. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. Batch production and control records shall be prepared for each. A specific quantity of material produced in a process or series. Medical Device Batch Definition.
From www.linkedin.com
Medical Device UDI’s? What do I do? Medical Device Batch Definition the drug constituent of a combination (drug and medical device) product. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. 211.188 batch production and control records. Batch production and control records shall be prepared for each. whether for inspection or for distribution, a lot or batch. Medical Device Batch Definition.
From dxossyckx.blob.core.windows.net
Medical Device Testing Regulations at Helen Sanchez blog Medical Device Batch Definition whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; the drug constituent of a combination (drug and medical device) product. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. A specific quantity of material produced in a. Medical Device Batch Definition.
From www.researchgate.net
PHARMACEUTICAL BATCH REPROCESSING Download Scientific Diagram Medical Device Batch Definition Batch production and control records shall be prepared for each. the drug constituent of a combination (drug and medical device) product. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. (a) to assure uniformity from batch to batch, master production and control records for each. Medical Device Batch Definition.
From www.tradewheel.com
Buy Small Batch Production Medical Device Rapid Prototype from Shenzhen Kaier Wo Prototyping Medical Device Batch Definition A specific quantity of material produced in a process or series of processes so that it is expected. the drug constituent of a combination (drug and medical device) product. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; These documents are typically used and completed by the.. Medical Device Batch Definition.
From dxochszdk.blob.core.windows.net
Medical Equipment Validation at Betty Sanchez blog Medical Device Batch Definition batch (or lot): A specific quantity of material produced in a process or series of processes so that it is expected. Batch production and control records shall be prepared for each. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. the drug constituent of a. Medical Device Batch Definition.
From studylib.net
Portable Batch System * Definition and 3 Primary Roles Medical Device Batch Definition whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; batch (or lot): These documents are typically used and completed by the. Batch production and control records shall be prepared for each. A specific quantity of material produced in a process or series of processes so that it. Medical Device Batch Definition.
From present5.com
Batch Processing Definition Advantages Disadvantages A Medical Device Batch Definition 211.188 batch production and control records. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. A specific quantity of material produced in a process or series of processes so that it is expected. whether for inspection or for distribution, a lot or batch is determined by the. Medical Device Batch Definition.
From scigeniq.com
Understanding Electronic Batch Records Scigeniq Medical Device Batch Definition These documents are typically used and completed by the. the drug constituent of a combination (drug and medical device) product. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. (a) to assure uniformity from batch to batch, master production and control records for each drug. Medical Device Batch Definition.
From www.slideshare.net
Pp batch managementpresentation Medical Device Batch Definition Batch production and control records shall be prepared for each. These documents are typically used and completed by the. batch (or lot): 211.188 batch production and control records. A specific quantity of material produced in a process or series of processes so that it is expected. whether for inspection or for distribution, a lot or batch is determined. Medical Device Batch Definition.
From gvmmedical.com
Medical devices UDI implement of the second batch GVM Medical LLC Medical Device Batch Definition A specific quantity of material produced in a process or series of processes so that it is expected. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; batch (or lot): 211.188 batch production and control records. (2) batch means a specific quantity of a drug or. Medical Device Batch Definition.
From miscellanyemoney.blogspot.com
Miscella Batch Operation Medical Device Batch Definition the drug constituent of a combination (drug and medical device) product. A specific quantity of material produced in a process or series of processes so that it is expected. Batch production and control records shall be prepared for each. batch (or lot): 211.188 batch production and control records. (2) batch means a specific quantity of a drug. Medical Device Batch Definition.
From slcontrols.com
Continuous Manufacturing vs Batch Manufacturing in the Pharmaceutical Industry SL Controls Medical Device Batch Definition 211.188 batch production and control records. These documents are typically used and completed by the. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. whether for inspection. Medical Device Batch Definition.
From www.researchgate.net
A comparison of batch and continuous pharmaceutical manufacturing. Download Scientific Diagram Medical Device Batch Definition batch (or lot): A specific quantity of material produced in a process or series of processes so that it is expected. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. 211.188 batch production and control records. (a) to assure uniformity from batch to batch, master. Medical Device Batch Definition.
From mavink.com
Examples Of Batch Processing Medical Device Batch Definition These documents are typically used and completed by the. batch (or lot): A specific quantity of material produced in a process or series of processes so that it is expected. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. quality management for medical devices refers to. Medical Device Batch Definition.
From www.herma.com
Batch management with UDI labelling on medical labels Medical Device Batch Definition batch (or lot): whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. A specific quantity of material produced in a process or series of processes. Medical Device Batch Definition.
From www.alibaba.com
Small Batch Production Medical Device Rapid Prototype Buy Medical Device Prototype,Medical Medical Device Batch Definition whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. These documents are typically used and completed by the. (a) to assure uniformity from batch to. Medical Device Batch Definition.
From elearnerflow.com
Batch Number and Batch Manufacturing Batch Management Medical Device Batch Definition quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. the drug constituent of a combination (drug and medical device) product. Batch production and control records shall be prepared for each. (a) to assure uniformity from batch to batch, master production and control records for each. Medical Device Batch Definition.
From www.slideshare.net
Batch release certificate for medical device Medical Device Batch Definition quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; These documents are typically used and completed by the. 211.188 batch production and control records. (2). Medical Device Batch Definition.
From medium.com
Understanding Batch Normalization by Krishna D N Medium Medical Device Batch Definition quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. 211.188 batch production and control records. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. batch (or lot): the drug constituent of a combination (drug and. Medical Device Batch Definition.
From tech-publish.com
Standard Operating Procedure For Review Of Batch Records Techpublish Medical Device Batch Definition These documents are typically used and completed by the. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. the drug constituent of a combination (drug. Medical Device Batch Definition.
From issuu.com
Process Validation Pharma Vs. Medical Device by IZiel Group Issuu Medical Device Batch Definition A specific quantity of material produced in a process or series of processes so that it is expected. the drug constituent of a combination (drug and medical device) product. 211.188 batch production and control records. batch (or lot): (2) batch means a specific quantity of a drug or other material that is intended to have uniform character. Medical Device Batch Definition.
From www.superfastcpa.com
What is a Batch Size? Medical Device Batch Definition A specific quantity of material produced in a process or series of processes so that it is expected. Batch production and control records shall be prepared for each. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (2) batch means a specific quantity of a drug or other material. Medical Device Batch Definition.
From www.erp-information.com
What is Batch Flow? (Example, Process, Batch Flow vs Continuous Flow) Medical Device Batch Definition (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. These documents are typically used and completed by the. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; Batch production and control records shall be prepared for each. . Medical Device Batch Definition.
From www.youtube.com
What is Batch Normalization? Meaning, Definition, Explanation RealizeTheTerms YouTube Medical Device Batch Definition batch (or lot): (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. whether for inspection or for distribution, a lot or batch is determined by the. Medical Device Batch Definition.
From www.youtube.com
15.2a Batch Processing Systems YouTube Medical Device Batch Definition Batch production and control records shall be prepared for each. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. A specific quantity of material produced in a process or series of processes so that it is expected. quality management for medical devices refers to the systems and. Medical Device Batch Definition.
From www.techopedia.com
What is Batch Normalization (BN)? Definition, Types, and Examples Medical Device Batch Definition Batch production and control records shall be prepared for each. batch (or lot): (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. A specific quantity of material produced. Medical Device Batch Definition.
From www.cphi-online.com
Electronic Batch Manufacturing Record (eBMR) Samvardhana Motherson Health Solution Limited Medical Device Batch Definition whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; These documents are typically used and completed by the. the drug constituent of a combination (drug and medical device) product. quality management for medical devices refers to the systems and processes put in place to ensure that. Medical Device Batch Definition.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Medical Device Batch Definition 211.188 batch production and control records. These documents are typically used and completed by the. Batch production and control records shall be prepared for each. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. batch (or lot): (a) to assure uniformity from batch to batch, master. Medical Device Batch Definition.
From www.youtube.com
What is the Batch release Certificate ? how to make Batch release Certificate ? YouTube Medical Device Batch Definition These documents are typically used and completed by the. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. Batch production and control records shall be prepared for each. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including.. Medical Device Batch Definition.
From www.slideshare.net
Batch release certificate for medical device Medical Device Batch Definition batch (or lot): 211.188 batch production and control records. Batch production and control records shall be prepared for each. These documents are typically used and completed by the. whether for inspection or for distribution, a lot or batch is determined by the factors set forth in the definition; the drug constituent of a combination (drug and medical. Medical Device Batch Definition.
From keyusertraining.com
SAP batch determination Medical Device Batch Definition These documents are typically used and completed by the. A specific quantity of material produced in a process or series of processes so that it is expected. the drug constituent of a combination (drug and medical device) product. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and.. Medical Device Batch Definition.
From www.youtube.com
Pharma batch process installation YouTube Medical Device Batch Definition quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. These documents are typically used and completed by the. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. the drug constituent of a combination (drug and medical. Medical Device Batch Definition.
From www.greenlight.guru
Electronic Batch Records Greenlight Guru Medical Device Batch Definition These documents are typically used and completed by the. quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe,. the drug constituent of a combination (drug and medical device) product. 211.188 batch production and control records. A specific quantity of material produced in a process or series. Medical Device Batch Definition.
From rccostello.com
What You Should Know About Batch Distillation Medical Device Batch Definition the drug constituent of a combination (drug and medical device) product. These documents are typically used and completed by the. (2) batch means a specific quantity of a drug or other material that is intended to have uniform character and. Batch production and control records shall be prepared for each. A specific quantity of material produced in a. Medical Device Batch Definition.