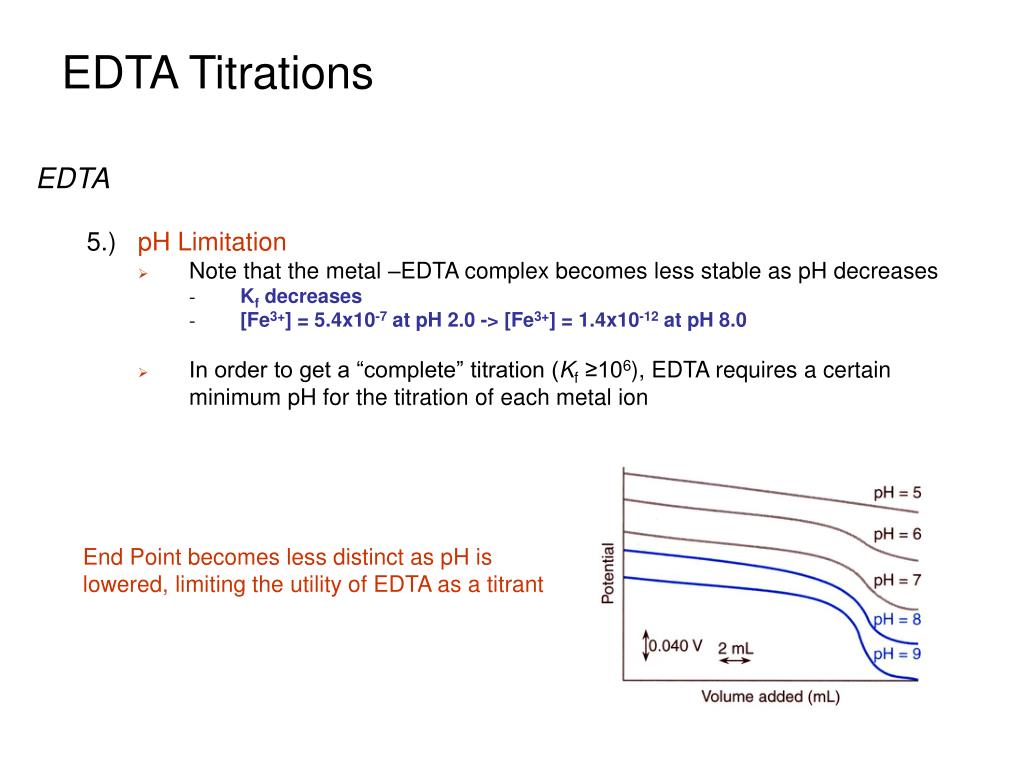
Limitations Of Edta Titration . Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Find out the properties, stability, and types. What are the limitations of complexometric titration? The most widely used universal. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Some limitations of complexometric titration include sensitivity to interfering. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Find out how to calculate the conditional formation. Chelators and end point indicators are the most important parts of complexometric titrations. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to.
from www.slideserve.com
Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Find out how to calculate the conditional formation. Some limitations of complexometric titration include sensitivity to interfering. Chelators and end point indicators are the most important parts of complexometric titrations. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. What are the limitations of complexometric titration? Find out the properties, stability, and types.
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499
Limitations Of Edta Titration Find out the properties, stability, and types. Find out how to calculate the conditional formation. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Some limitations of complexometric titration include sensitivity to interfering. Chelators and end point indicators are the most important parts of complexometric titrations. Find out the properties, stability, and types. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. What are the limitations of complexometric titration? The most widely used universal.
From mungfali.com
EDTA Titration Curve Limitations Of Edta Titration Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Find out the properties, stability, and types. What are the limitations of complexometric titration? The most widely used universal. Chelators and end point indicators are the most important parts of complexometric titrations. Find out how to calculate the conditional formation. Learn. Limitations Of Edta Titration.
From gbu-presnenskij.ru
EDTA Titration, Types, Advantages, Disadvantages, 54 OFF Limitations Of Edta Titration Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Chelators and end point indicators are the most important parts of complexometric titrations. Complexometric titrations using chelating agents like edta and its derivatives. Limitations Of Edta Titration.
From fyoqjwhlp.blob.core.windows.net
Titration Advantages And Disadvantages at Lera Burke blog Limitations Of Edta Titration Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. What are the limitations of complexometric titration? Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID234018 Limitations Of Edta Titration Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Chelators and end point. Limitations Of Edta Titration.
From gbu-presnenskij.ru
EDTA Titration, Types, Advantages, Disadvantages, 54 OFF Limitations Of Edta Titration Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Chelators and end point. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID1079499 Limitations Of Edta Titration Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Find out how to calculate the conditional formation. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many. Limitations Of Edta Titration.
From www.slideserve.com
PPT TYBSc Paper IV USCH604 Analytical Chemistry COMPLEXOMETRIC Limitations Of Edta Titration Some limitations of complexometric titration include sensitivity to interfering. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount. Limitations Of Edta Titration.
From brunofuga.adv.br
Complexometric Titrations Types, Indicators, Advantages,, 41 OFF Limitations Of Edta Titration Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Some limitations of complexometric titration include sensitivity to interfering. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titration of Water PowerPoint Presentation, free download Limitations Of Edta Titration Chelators and end point indicators are the most important parts of complexometric titrations. Find out how to calculate the conditional formation. The most widely used universal. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Some limitations of complexometric titration include sensitivity to interfering. What are the limitations of complexometric. Limitations Of Edta Titration.
From brunofuga.adv.br
EDTA Titration, Types, Advantages, Disadvantages, 52 OFF Limitations Of Edta Titration Find out the properties, stability, and types. What are the limitations of complexometric titration? Find out how to calculate the conditional formation. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. The most widely used universal. In comparison of advanced instrumental techniques, the edta titration is simple to operate,. Limitations Of Edta Titration.
From www.studypool.com
SOLUTION Edta titration calculations Studypool Limitations Of Edta Titration Find out the properties, stability, and types. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Find out how to calculate the conditional formation. The most widely used universal. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Chelators and end. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID1079499 Limitations Of Edta Titration What are the limitations of complexometric titration? Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Chelators and end point indicators are the most important parts of complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Learn how to use. Limitations Of Edta Titration.
From slideplayer.com
Name Dr. Pramod B. Thakur Class T. Y. B ppt download Limitations Of Edta Titration In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. What are the limitations of complexometric titration? Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Find out how to calculate the conditional formation. Chelators and end point indicators are. Limitations Of Edta Titration.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Limitations Of Edta Titration Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Find out how to calculate the conditional formation. Chelators and end point indicators are the most important parts of complexometric titrations. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Limitations Of Edta Titration The most widely used universal. What are the limitations of complexometric titration? Find out the properties, stability, and types. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn about the principles and. Limitations Of Edta Titration.
From slidetodoc.com
EDTA Titrations Introduction 1 Metal Chelate Complexes Any Limitations Of Edta Titration Find out the properties, stability, and types. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Some limitations of complexometric titration include sensitivity to interfering. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Chelators and end point indicators. Limitations Of Edta Titration.
From scienceinfo.com
EDTA Titration, Types, Advantages, Disadvantages Limitations Of Edta Titration Find out the properties, stability, and types. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with. Limitations Of Edta Titration.
From www.slideshare.net
Complexometric titration Limitations Of Edta Titration Find out how to calculate the conditional formation. Chelators and end point indicators are the most important parts of complexometric titrations. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. What. Limitations Of Edta Titration.
From slidetodoc.com
Lab 11 EDTA Titration of Ca 2 and Limitations Of Edta Titration Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Some limitations of complexometric titration include sensitivity to interfering. The most widely used universal. Learn about the principles. Limitations Of Edta Titration.
From mungfali.com
EDTA Titration Curve Limitations Of Edta Titration What are the limitations of complexometric titration? Find out how to calculate the conditional formation. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn how to calculate the fractional and conditional formation constants of. Limitations Of Edta Titration.
From slidetodoc.com
Chapter 12 EDTA Titrations Overview 12 1 MetalChelate Limitations Of Edta Titration Find out the properties, stability, and types. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Some limitations of complexometric titration include sensitivity to interfering. Learn about the chemistry and. Limitations Of Edta Titration.
From v-s.mobi
Download Complexometric titration B pharma 1st sem Ph. Analysis Limitations Of Edta Titration Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. What are the limitations of complexometric titration? Find out how to calculate the conditional formation. Learn about the principles and applications of complexation. Limitations Of Edta Titration.
From slideplayer.com
Complexometric titration Dr.Bhagure G.R. ppt download Limitations Of Edta Titration The most widely used universal. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Chelators and end point indicators are the most important parts of complexometric titrations. Find out the properties, stability, and types. Find out how to calculate the conditional formation. What are the limitations of complexometric titration? Learn how to. Limitations Of Edta Titration.
From scienceinfo.com
EDTA Titration, Types, Advantages, Disadvantages Limitations Of Edta Titration Some limitations of complexometric titration include sensitivity to interfering. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Learn how to use edta, a chelating agent, in complexometric titration to. Limitations Of Edta Titration.
From scienceinfo.com
EDTA Titration, Types, Advantages, Disadvantages Limitations Of Edta Titration Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Find out the properties, stability, and types. What are the limitations of complexometric titration? Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Complexometric titrations using chelating agents like edta and its. Limitations Of Edta Titration.
From dokumen.tips
(PDF) Continuous EDTA Titrations at Low Concentrations DOKUMEN.TIPS Limitations Of Edta Titration Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. Chelators and end point indicators are the most important parts of complexometric titrations. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Find out how to calculate the conditional formation.. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Limitations Of Edta Titration Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Chelators and end point indicators are the most important parts of complexometric titrations. Learn about the chemistry and properties of edta, a multidentate ligand. Limitations Of Edta Titration.
From mungfali.com
EDTA Titration Curve Limitations Of Edta Titration Some limitations of complexometric titration include sensitivity to interfering. Chelators and end point indicators are the most important parts of complexometric titrations. Find out how to calculate the conditional formation. The most widely used universal. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Complexometric titrations using chelating agents like edta and. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titration of Water PowerPoint Presentation, free download Limitations Of Edta Titration Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Find out the properties, stability, and types. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal. Limitations Of Edta Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Limitations Of Edta Titration Find out the properties, stability, and types. Some limitations of complexometric titration include sensitivity to interfering. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. The most widely used universal.. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Limitations Of Edta Titration Learn about the chemistry and properties of edta, a multidentate ligand that forms stable 1:1 complexes with many metal. Some limitations of complexometric titration include sensitivity to interfering. Find out the properties, stability, and types. Learn how to calculate the fractional and conditional formation constants of edta and its metal complexes, and how to use them to. The most widely. Limitations Of Edta Titration.
From www.scribd.com
EDTA Titration PDF Titration Chemistry Limitations Of Edta Titration Some limitations of complexometric titration include sensitivity to interfering. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn how to calculate the fractional and conditional formation constants of edta and its metal. Limitations Of Edta Titration.
From slidetodoc.com
Chapter 12 EDTA Titrations Overview 12 1 MetalChelate Limitations Of Edta Titration In comparison of advanced instrumental techniques, the edta titration is simple to operate, can produce accurate results. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn about the principles and applications of complexation titrations, especially the use of edta as a ligand. Learn about the chemistry and properties of. Limitations Of Edta Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Limitations Of Edta Titration Find out the properties, stability, and types. Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. Learn how to calculate the fractional and conditional formation constants of edta and its. Limitations Of Edta Titration.
From slideplayer.com
Experiments in Analytical Chemistry EDTA determination of Ca and Mg in Limitations Of Edta Titration Complexometric titrations using chelating agents like edta and its derivatives have limitations due to their influence on the titration. Find out how to calculate the conditional formation. Learn how to use edta, a chelating agent, in complexometric titration to determine the amount of metal ions in solution. In comparison of advanced instrumental techniques, the edta titration is simple to operate,. Limitations Of Edta Titration.