Electrode Definition Redox . When a substance loses an electron, its oxidation state increases; Interpret electrode potentials in terms of relative oxidant and reductant strengths. The redox potential is used to describe a system's overall reducing or oxidizing. When the current leaves the electrodes it is. Describe and relate the definitions of electrode and cell potentials. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. This page explains the background to standard electrode potentials. An electrode by definition is a point where current enters and leaves the electrolyte. What is the unit of redox potential? Oxidation is the process of electron loss: Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. An introduction to redox equilibria and electrode potentials. What are the types of electrode potential?
from www.aakash.ac.in
The redox potential is used to describe a system's overall reducing or oxidizing. Describe and relate the definitions of electrode and cell potentials. An introduction to redox equilibria and electrode potentials. When a substance loses an electron, its oxidation state increases; When the current leaves the electrodes it is. What is the unit of redox potential? This page explains the background to standard electrode potentials. An electrode by definition is a point where current enters and leaves the electrolyte. Interpret electrode potentials in terms of relative oxidant and reductant strengths. A redox reaction is a reaction that involves a change in oxidation state of one or more elements.
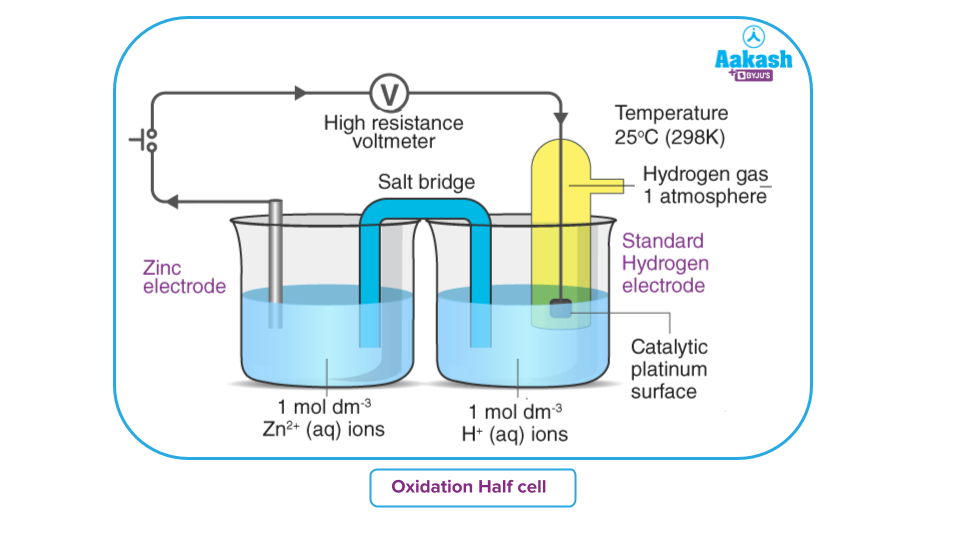
Standard Electrode Potential Importance, Definition & Redox Reactions
Electrode Definition Redox The redox potential is used to describe a system's overall reducing or oxidizing. The redox potential is used to describe a system's overall reducing or oxidizing. An introduction to redox equilibria and electrode potentials. What are the types of electrode potential? Describe and relate the definitions of electrode and cell potentials. When the current leaves the electrodes it is. Oxidation is the process of electron loss: This page explains the background to standard electrode potentials. When a substance loses an electron, its oxidation state increases; Interpret electrode potentials in terms of relative oxidant and reductant strengths. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. An electrode by definition is a point where current enters and leaves the electrolyte. What is the unit of redox potential? Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential.
From slideplayer.com
What is an Electrochemical Cell? ppt download Electrode Definition Redox When a substance loses an electron, its oxidation state increases; Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. This page explains the background to standard electrode potentials. When the current leaves the electrodes it is. An introduction to redox equilibria and electrode potentials. What are the types. Electrode Definition Redox.
From slideplayer.com
ELECTRODE PROCESSES, THEIR BIOLOGICAL ROLE AND USE IN MEDICINE ppt Electrode Definition Redox A redox reaction is a reaction that involves a change in oxidation state of one or more elements. When the current leaves the electrodes it is. An electrode by definition is a point where current enters and leaves the electrolyte. What are the types of electrode potential? This page explains the background to standard electrode potentials. Electrochemical cells permit this. Electrode Definition Redox.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. When the current leaves the electrodes it is. Oxidation is the process of electron loss: Describe and relate the definitions of electrode and cell potentials. What are the types of electrode potential? An electrode by definition is a point where current enters and leaves the electrolyte. When a substance. Electrode Definition Redox.
From saylordotorg.github.io
Applications of Redox Reactions Voltaic Cells Electrode Definition Redox A redox reaction is a reaction that involves a change in oxidation state of one or more elements. Interpret electrode potentials in terms of relative oxidant and reductant strengths. This page explains the background to standard electrode potentials. When a substance loses an electron, its oxidation state increases; An electrode by definition is a point where current enters and leaves. Electrode Definition Redox.
From www.thoughtco.com
How to Define Anode and Cathode Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. Oxidation is the process of electron loss: Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. What are the types of electrode potential? An introduction to redox equilibria and electrode potentials. When a substance loses an electron, its oxidation state increases; A. Electrode Definition Redox.
From www.youtube.com
A short guide to redox electrodes YouTube Electrode Definition Redox This page explains the background to standard electrode potentials. Oxidation is the process of electron loss: When a substance loses an electron, its oxidation state increases; A redox reaction is a reaction that involves a change in oxidation state of one or more elements. What is the unit of redox potential? An introduction to redox equilibria and electrode potentials. An. Electrode Definition Redox.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Electrode Definition Redox A redox reaction is a reaction that involves a change in oxidation state of one or more elements. An introduction to redox equilibria and electrode potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. What is the unit of redox potential? When the current leaves the electrodes it is. Electrochemical cells permit this relative redox activity to. Electrode Definition Redox.
From www.aakash.ac.in
Standard Electrode Potential Importance, Definition & Redox Reactions Electrode Definition Redox This page explains the background to standard electrode potentials. Oxidation is the process of electron loss: Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. When the current leaves the electrodes it is. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. An electrode. Electrode Definition Redox.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Electrode Definition Redox This page explains the background to standard electrode potentials. Oxidation is the process of electron loss: What are the types of electrode potential? When the current leaves the electrodes it is. An introduction to redox equilibria and electrode potentials. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. What is the. Electrode Definition Redox.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Electrode Definition Redox When a substance loses an electron, its oxidation state increases; What is the unit of redox potential? Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. This page explains the background to standard electrode potentials. The redox potential is used to describe a system's overall reducing or oxidizing. Describe and relate the definitions. Electrode Definition Redox.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. What are the types of electrode potential? Oxidation is the process of electron loss: An electrode by definition is a point where current enters and leaves the electrolyte. This page explains. Electrode Definition Redox.
From www.chemistrylearner.com
Redox (OxidationReduction) Reaction Definition & Examples Electrode Definition Redox When the current leaves the electrodes it is. Describe and relate the definitions of electrode and cell potentials. The redox potential is used to describe a system's overall reducing or oxidizing. This page explains the background to standard electrode potentials. What is the unit of redox potential? Electrochemical cells permit this relative redox activity to be quantified by an easily. Electrode Definition Redox.
From www.slideserve.com
PPT Chemical equilibrium electrochemistry PowerPoint Presentation Electrode Definition Redox An introduction to redox equilibria and electrode potentials. Oxidation is the process of electron loss: What are the types of electrode potential? Interpret electrode potentials in terms of relative oxidant and reductant strengths. When a substance loses an electron, its oxidation state increases; Describe and relate the definitions of electrode and cell potentials. An electrode by definition is a point. Electrode Definition Redox.
From www.kgs.ku.edu
Untitled Document [www.kgs.ku.edu] Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. An electrode by definition is a point where current enters and leaves the electrolyte. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. When the current leaves the electrodes it is. When. Electrode Definition Redox.
From alevelchemistry.co.uk
Redox and Electrode Potential ALevel Chemistry Revision Notes Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. An introduction to redox equilibria and electrode potentials. The redox potential is used to describe a system's overall reducing or oxidizing. An electrode by definition is a point where current enters and leaves the electrolyte. A redox reaction is a reaction that involves a change in oxidation state of. Electrode Definition Redox.
From www.youtube.com
A2 Chemistry Redox Equilibria , Definition of Standard Electrode Electrode Definition Redox When the current leaves the electrodes it is. Describe and relate the definitions of electrode and cell potentials. What is the unit of redox potential? An electrode by definition is a point where current enters and leaves the electrolyte. Oxidation is the process of electron loss: What are the types of electrode potential? When a substance loses an electron, its. Electrode Definition Redox.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Standard Electrode Potential Eo (1) Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Oxidation is the process of electron loss: An electrode by definition is a point where current enters and leaves the electrolyte. An introduction to redox equilibria and electrode potentials. What are the types of electrode potential? When a substance. Electrode Definition Redox.
From slidetodoc.com
Derivation of Nernst Equation for Single electrode Consider Electrode Definition Redox When a substance loses an electron, its oxidation state increases; An introduction to redox equilibria and electrode potentials. When the current leaves the electrodes it is. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The redox potential is used to describe a system's overall reducing or oxidizing. A redox reaction is a reaction that involves a change. Electrode Definition Redox.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Electrode Definition Redox The redox potential is used to describe a system's overall reducing or oxidizing. What is the unit of redox potential? When the current leaves the electrodes it is. Describe and relate the definitions of electrode and cell potentials. This page explains the background to standard electrode potentials. An electrode by definition is a point where current enters and leaves the. Electrode Definition Redox.
From www.youtube.com
REDOX REACTIONS AND ELECTRODE PROCESSES YouTube Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. This page explains the background to standard electrode potentials. When the current leaves the electrodes it is. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. An introduction to redox equilibria and electrode potentials. When a substance loses an electron,. Electrode Definition Redox.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Electrode Definition Redox An electrode by definition is a point where current enters and leaves the electrolyte. The redox potential is used to describe a system's overall reducing or oxidizing. What are the types of electrode potential? Interpret electrode potentials in terms of relative oxidant and reductant strengths. What is the unit of redox potential? When the current leaves the electrodes it is.. Electrode Definition Redox.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID Electrode Definition Redox Describe and relate the definitions of electrode and cell potentials. This page explains the background to standard electrode potentials. When a substance loses an electron, its oxidation state increases; The redox potential is used to describe a system's overall reducing or oxidizing. Interpret electrode potentials in terms of relative oxidant and reductant strengths. When the current leaves the electrodes it. Electrode Definition Redox.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Electrode Definition Redox Describe and relate the definitions of electrode and cell potentials. An introduction to redox equilibria and electrode potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. An electrode by definition is a point where current enters and leaves the electrolyte. What are the types of electrode potential? The redox potential is used to describe a system's overall. Electrode Definition Redox.
From merinyjoreenmatias.weebly.com
TASK 2 OXIDATION AND REDUCTION PRINCIPLES IN BIOCHEMISTRY Electrode Definition Redox Interpret electrode potentials in terms of relative oxidant and reductant strengths. An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it is. The redox potential is used to describe a system's overall reducing or oxidizing. What is the unit of redox potential? Electrochemical cells permit this relative redox activity. Electrode Definition Redox.
From www.studypool.com
SOLUTION Redox reactions part 2 Studypool Electrode Definition Redox The redox potential is used to describe a system's overall reducing or oxidizing. Oxidation is the process of electron loss: When a substance loses an electron, its oxidation state increases; This page explains the background to standard electrode potentials. Describe and relate the definitions of electrode and cell potentials. An electrode by definition is a point where current enters and. Electrode Definition Redox.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrode Definition Redox When a substance loses an electron, its oxidation state increases; An introduction to redox equilibria and electrode potentials. Oxidation is the process of electron loss: An electrode by definition is a point where current enters and leaves the electrolyte. Interpret electrode potentials in terms of relative oxidant and reductant strengths. When the current leaves the electrodes it is. A redox. Electrode Definition Redox.
From www.youtube.com
Difference between Oxidation potential and Reduction potential Electrode Definition Redox What is the unit of redox potential? Interpret electrode potentials in terms of relative oxidant and reductant strengths. A redox reaction is a reaction that involves a change in oxidation state of one or more elements. What are the types of electrode potential? Oxidation is the process of electron loss: When the current leaves the electrodes it is. An introduction. Electrode Definition Redox.
From www.aakash.ac.in
Standard Electrode Potential Importance, Definition & Redox Reactions Electrode Definition Redox An introduction to redox equilibria and electrode potentials. The redox potential is used to describe a system's overall reducing or oxidizing. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. A redox. Electrode Definition Redox.
From www.aakash.ac.in
Redox Reactions in Electrodes Redox Reactions, Electrodes, Redox Electrode Definition Redox What is the unit of redox potential? Describe and relate the definitions of electrode and cell potentials. This page explains the background to standard electrode potentials. An electrode by definition is a point where current enters and leaves the electrolyte. Interpret electrode potentials in terms of relative oxidant and reductant strengths. An introduction to redox equilibria and electrode potentials. What. Electrode Definition Redox.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Electrode Definition Redox What is the unit of redox potential? What are the types of electrode potential? The redox potential is used to describe a system's overall reducing or oxidizing. An introduction to redox equilibria and electrode potentials. Oxidation is the process of electron loss: When a substance loses an electron, its oxidation state increases; This page explains the background to standard electrode. Electrode Definition Redox.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Electrode Definition Redox An electrode by definition is a point where current enters and leaves the electrolyte. What are the types of electrode potential? When the current leaves the electrodes it is. This page explains the background to standard electrode potentials. When a substance loses an electron, its oxidation state increases; A redox reaction is a reaction that involves a change in oxidation. Electrode Definition Redox.
From www.vedantu.com
Types of Redox Reactions Important Concepts and Tips for JEE Electrode Definition Redox An electrode by definition is a point where current enters and leaves the electrolyte. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. An introduction to redox equilibria and electrode potentials. A redox reaction is a reaction that involves a change in oxidation. Electrode Definition Redox.
From www.aakash.ac.in
Redox Reactions in Electrodes Redox Reactions, Electrodes, Redox Electrode Definition Redox What are the types of electrode potential? When a substance loses an electron, its oxidation state increases; An electrode by definition is a point where current enters and leaves the electrolyte. The redox potential is used to describe a system's overall reducing or oxidizing. Oxidation is the process of electron loss: When the current leaves the electrodes it is. Describe. Electrode Definition Redox.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Electrode Definition Redox A redox reaction is a reaction that involves a change in oxidation state of one or more elements. When the current leaves the electrodes it is. This page explains the background to standard electrode potentials. Oxidation is the process of electron loss: Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. What are. Electrode Definition Redox.
From mungfali.com
Types Of Electrodes Electrode Definition Redox An electrode by definition is a point where current enters and leaves the electrolyte. What is the unit of redox potential? An introduction to redox equilibria and electrode potentials. Oxidation is the process of electron loss: When the current leaves the electrodes it is. Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential.. Electrode Definition Redox.