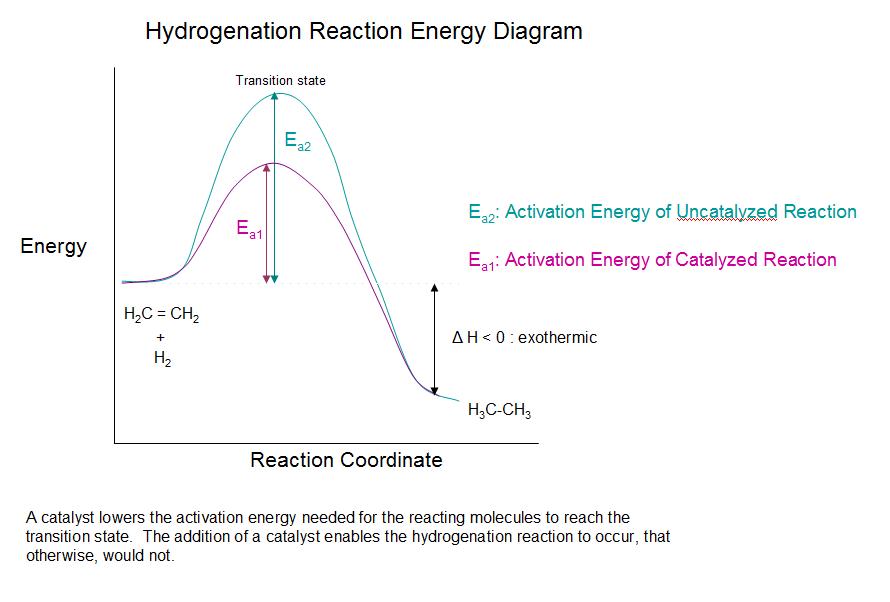
Catalyst Chemical Reaction Activation Energy . This means that the reactant molecules have enough kinetic energy to. It does not lower the activation energy of the reaction. There is a subtle difference between the. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It includes the activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The activation energy is shown as a 'hump' in the line, which: However, it does increase the frequency of. A catalyst provides an alternative route for the reaction with a lower activation energy. The key importance of activation energy. Reactants often get activation energy from heat, but sometimes. Starts at the energy of. This does not change the frequency of collisions. The activation energy is the minimum energy required for a reaction to occur.
from chem.libretexts.org
This does not change the frequency of collisions. The activation energy is the minimum energy required for a reaction to occur. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The key importance of activation energy. Reactants often get activation energy from heat, but sometimes. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. However, it does increase the frequency of. This means that the reactant molecules have enough kinetic energy to. It includes the activation energy. The activation energy is shown as a 'hump' in the line, which:
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts
Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. The key importance of activation energy. The activation energy is the minimum energy required for a reaction to occur. Reactants often get activation energy from heat, but sometimes. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It does not lower the activation energy of the reaction. This does not change the frequency of collisions. Starts at the energy of. There is a subtle difference between the. It includes the activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. This means that the reactant molecules have enough kinetic energy to. However, it does increase the frequency of. The activation energy is shown as a 'hump' in the line, which:
From www.slideserve.com
PPT KEY CONCEPT Enzymes are catalysts for chemical reactions in Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. However, it does increase the frequency of. It does not lower the activation energy of the reaction. Starts at the energy of. A catalyst provides an alternative route for the reaction with a lower activation energy. This does not change the frequency of collisions. The key importance of activation energy. The activation energy. Catalyst Chemical Reaction Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Chemical Reaction Activation Energy However, it does increase the frequency of. The activation energy is shown as a 'hump' in the line, which: It does not lower the activation energy of the reaction. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. In. Catalyst Chemical Reaction Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Chemical Reaction Activation Energy However, it does increase the frequency of. The activation energy is the minimum energy required for a reaction to occur. The activation energy is shown as a 'hump' in the line, which: Reactants often get activation energy from heat, but sometimes. Starts at the energy of. Collisions only result in a reaction if the particles collide with a certain minimum. Catalyst Chemical Reaction Activation Energy.
From joivqpwri.blob.core.windows.net
Catalyst In Chemistry Class 10 at Eric Dougherty blog Catalyst Chemical Reaction Activation Energy The activation energy is the minimum energy required for a reaction to occur. There is a subtle difference between the. It does not lower the activation energy of the reaction. However, it does increase the frequency of. The key importance of activation energy. It includes the activation energy. A catalyst provides an alternative route for the reaction with a lower. Catalyst Chemical Reaction Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalyst Chemical Reaction Activation Energy A catalyst provides an alternative route for the reaction with a lower activation energy. The key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. This means that the reactant molecules have enough kinetic energy to. The activation energy is the minimum energy. Catalyst Chemical Reaction Activation Energy.
From slideplayer.com
Enzymes. ppt download Catalyst Chemical Reaction Activation Energy Reactants often get activation energy from heat, but sometimes. It does not lower the activation energy of the reaction. The activation energy is shown as a 'hump' in the line, which: Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The activation energy is the minimum energy. Catalyst Chemical Reaction Activation Energy.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. The key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. The activation energy is shown as a 'hump' in the line,. Catalyst Chemical Reaction Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Chemical Reaction Activation Energy Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of. This does not change the frequency of collisions. A catalyst provides an alternative route for the reaction with a lower activation energy. Reactants often get activation energy from heat, but sometimes.. Catalyst Chemical Reaction Activation Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Chemical Reaction Activation Energy It does not lower the activation energy of the reaction. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The activation energy is shown as a 'hump' in the line, which: The key importance of activation energy. There is a subtle difference between the. Starts at the energy of. The activation. Catalyst Chemical Reaction Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. A catalyst provides an alternative route for the reaction with a lower activation energy. This means that the reactant molecules have enough kinetic energy to. The activation energy is shown as a 'hump' in the line, which: However, it does increase the frequency of. Reactants often get activation energy from heat, but sometimes.. Catalyst Chemical Reaction Activation Energy.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Chemical Reaction Activation Energy It does not lower the activation energy of the reaction. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Reactants often get activation energy from heat, but sometimes. It. Catalyst Chemical Reaction Activation Energy.
From www.researchgate.net
Chemical Reaction Energy Profile Catalysts reduce the activation energy Catalyst Chemical Reaction Activation Energy This does not change the frequency of collisions. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The key importance of activation energy. There is a subtle difference between the. Reactants often get activation energy from heat, but sometimes. The activation energy is shown as a 'hump' in the line, which:. Catalyst Chemical Reaction Activation Energy.
From 2012books.lardbucket.org
Catalysis Catalyst Chemical Reaction Activation Energy It does not lower the activation energy of the reaction. It includes the activation energy. Reactants often get activation energy from heat, but sometimes. A catalyst provides an alternative route for the reaction with a lower activation energy. This means that the reactant molecules have enough kinetic energy to. The activation energy is the minimum energy required for a reaction. Catalyst Chemical Reaction Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalyst Chemical Reaction Activation Energy It includes the activation energy. The activation energy is the minimum energy required for a reaction to occur. The activation energy is shown as a 'hump' in the line, which: Reactants often get activation energy from heat, but sometimes. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the. Catalyst Chemical Reaction Activation Energy.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Chemical Reaction Activation Energy It includes the activation energy. However, it does increase the frequency of. There is a subtle difference between the. A catalyst provides an alternative route for the reaction with a lower activation energy. The activation energy is shown as a 'hump' in the line, which: This does not change the frequency of collisions. It does not lower the activation energy. Catalyst Chemical Reaction Activation Energy.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. Starts at the energy of. It does not lower the activation energy of the reaction. This means that the reactant molecules have enough kinetic energy to. This does not change the frequency of collisions. The activation energy is the minimum energy required for a reaction to occur. In chemistry and physics, activation energy. Catalyst Chemical Reaction Activation Energy.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Catalyst Chemical Reaction Activation Energy Reactants often get activation energy from heat, but sometimes. The activation energy is the minimum energy required for a reaction to occur. This means that the reactant molecules have enough kinetic energy to. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. It does not lower the. Catalyst Chemical Reaction Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Chemical Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat, but sometimes. The activation energy is the minimum energy required for a reaction to occur. A catalyst provides an alternative route for the reaction with a lower activation energy. It includes the activation energy. This. Catalyst Chemical Reaction Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Chemical Reaction Activation Energy Starts at the energy of. There is a subtle difference between the. The activation energy is the minimum energy required for a reaction to occur. Reactants often get activation energy from heat, but sometimes. A catalyst provides an alternative route for the reaction with a lower activation energy. It includes the activation energy. Collisions only result in a reaction if. Catalyst Chemical Reaction Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Chemical Reaction Activation Energy It includes the activation energy. Starts at the energy of. However, it does increase the frequency of. There is a subtle difference between the. This does not change the frequency of collisions. It does not lower the activation energy of the reaction. The activation energy is the minimum energy required for a reaction to occur. The key importance of activation. Catalyst Chemical Reaction Activation Energy.
From www.slideserve.com
PPT Chapter 6, Section 3 Pages 234239. PowerPoint Presentation, free Catalyst Chemical Reaction Activation Energy There is a subtle difference between the. The activation energy is the minimum energy required for a reaction to occur. The key importance of activation energy. The activation energy is shown as a 'hump' in the line, which: Reactants often get activation energy from heat, but sometimes. However, it does increase the frequency of. Collisions only result in a reaction. Catalyst Chemical Reaction Activation Energy.
From philschatz.com
Catalysis · Chemistry Catalyst Chemical Reaction Activation Energy It includes the activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. This means that the reactant molecules have enough kinetic energy to. Starts at the energy of. Reactants often get activation energy from heat, but sometimes. Collisions only result in a reaction if the particles collide with a certain minimum energy called. Catalyst Chemical Reaction Activation Energy.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemical Reaction Activation Energy The activation energy is the minimum energy required for a reaction to occur. Reactants often get activation energy from heat, but sometimes. Starts at the energy of. The activation energy is shown as a 'hump' in the line, which: There is a subtle difference between the. A catalyst provides an alternative route for the reaction with a lower activation energy.. Catalyst Chemical Reaction Activation Energy.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Chemical Reaction Activation Energy However, it does increase the frequency of. It includes the activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. The key importance of activation energy. This means that the reactant molecules have enough kinetic energy to. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical. Catalyst Chemical Reaction Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Chemical Reaction Activation Energy A catalyst provides an alternative route for the reaction with a lower activation energy. Reactants often get activation energy from heat, but sometimes. This does not change the frequency of collisions. It includes the activation energy. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Collisions only result in a reaction. Catalyst Chemical Reaction Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Chemical Reaction Activation Energy Reactants often get activation energy from heat, but sometimes. There is a subtle difference between the. This means that the reactant molecules have enough kinetic energy to. This does not change the frequency of collisions. However, it does increase the frequency of. A catalyst provides an alternative route for the reaction with a lower activation energy. The key importance of. Catalyst Chemical Reaction Activation Energy.
From www.visionlearning.com
Chemical Reactions II Process of Science Visionlearning Catalyst Chemical Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Starts at the energy of. This does not change the frequency of collisions. A catalyst provides an alternative route for the reaction with a lower activation energy. However, it does increase the frequency of. The activation energy is the minimum energy required. Catalyst Chemical Reaction Activation Energy.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Chemical Reaction Activation Energy The activation energy is the minimum energy required for a reaction to occur. Starts at the energy of. Reactants often get activation energy from heat, but sometimes. It includes the activation energy. The key importance of activation energy. There is a subtle difference between the. However, it does increase the frequency of. A catalyst provides an alternative route for the. Catalyst Chemical Reaction Activation Energy.
From professional.patrickneyman.com
Activation Energy Equation Catalyst Chemical Reaction Activation Energy This means that the reactant molecules have enough kinetic energy to. The key importance of activation energy. There is a subtle difference between the. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. This does not change the frequency of collisions. The activation energy is shown as a 'hump' in the. Catalyst Chemical Reaction Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Chemical Reaction Activation Energy Reactants often get activation energy from heat, but sometimes. It does not lower the activation energy of the reaction. There is a subtle difference between the. Starts at the energy of. The activation energy is shown as a 'hump' in the line, which: It includes the activation energy. This means that the reactant molecules have enough kinetic energy to. In. Catalyst Chemical Reaction Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalyst Chemical Reaction Activation Energy The key importance of activation energy. This means that the reactant molecules have enough kinetic energy to. The activation energy is shown as a 'hump' in the line, which: It does not lower the activation energy of the reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the. Catalyst Chemical Reaction Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Chemical Reaction Activation Energy This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of. Reactants often get activation energy from heat, but sometimes. This means that the reactant molecules have enough kinetic energy to. In chemistry and. Catalyst Chemical Reaction Activation Energy.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Chemical Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. This does not change the frequency of collisions. The activation energy is shown as a 'hump' in the line, which: A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of. Catalyst Chemical Reaction Activation Energy.
From studyonline.blog
What Is Activation Energy? Definition and Examples Catalyst Chemical Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It includes the activation energy. The activation energy is shown as a 'hump' in the line, which: The activation energy is the minimum energy required for a reaction to occur. This does not change the frequency of collisions. A catalyst provides an. Catalyst Chemical Reaction Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Chemical Reaction Activation Energy However, it does increase the frequency of. A catalyst provides an alternative route for the reaction with a lower activation energy. The activation energy is the minimum energy required for a reaction to occur. This means that the reactant molecules have enough kinetic energy to. Reactants often get activation energy from heat, but sometimes. The activation energy is shown as. Catalyst Chemical Reaction Activation Energy.