Do Molecules Change Shape . Molecules of different shapes can adapt with their corresponding bond angles. This shape is dependent on the preferred spatial orientation of covalent bonds to. Shapes of molecules & ions. Whether they are flat structures. How does molecule shape change with different numbers of bonds and electron pairs? As a result, the molecule forms a regular geometric shape. Then, compare the model to real. According to vsepr theory, a molecule is designated by the. Find out by adding single, double or triple bonds and lone pairs to the central atom. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Molecules can adapt the following shapes and bond angles: The three dimensional shape or configuration of a molecule is an important characteristic.
from www.snexplores.org
Molecules can adapt the following shapes and bond angles: How does molecule shape change with different numbers of bonds and electron pairs? This shape is dependent on the preferred spatial orientation of covalent bonds to. The three dimensional shape or configuration of a molecule is an important characteristic. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Molecules of different shapes can adapt with their corresponding bond angles. Then, compare the model to real. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Whether they are flat structures. Shapes of molecules & ions.
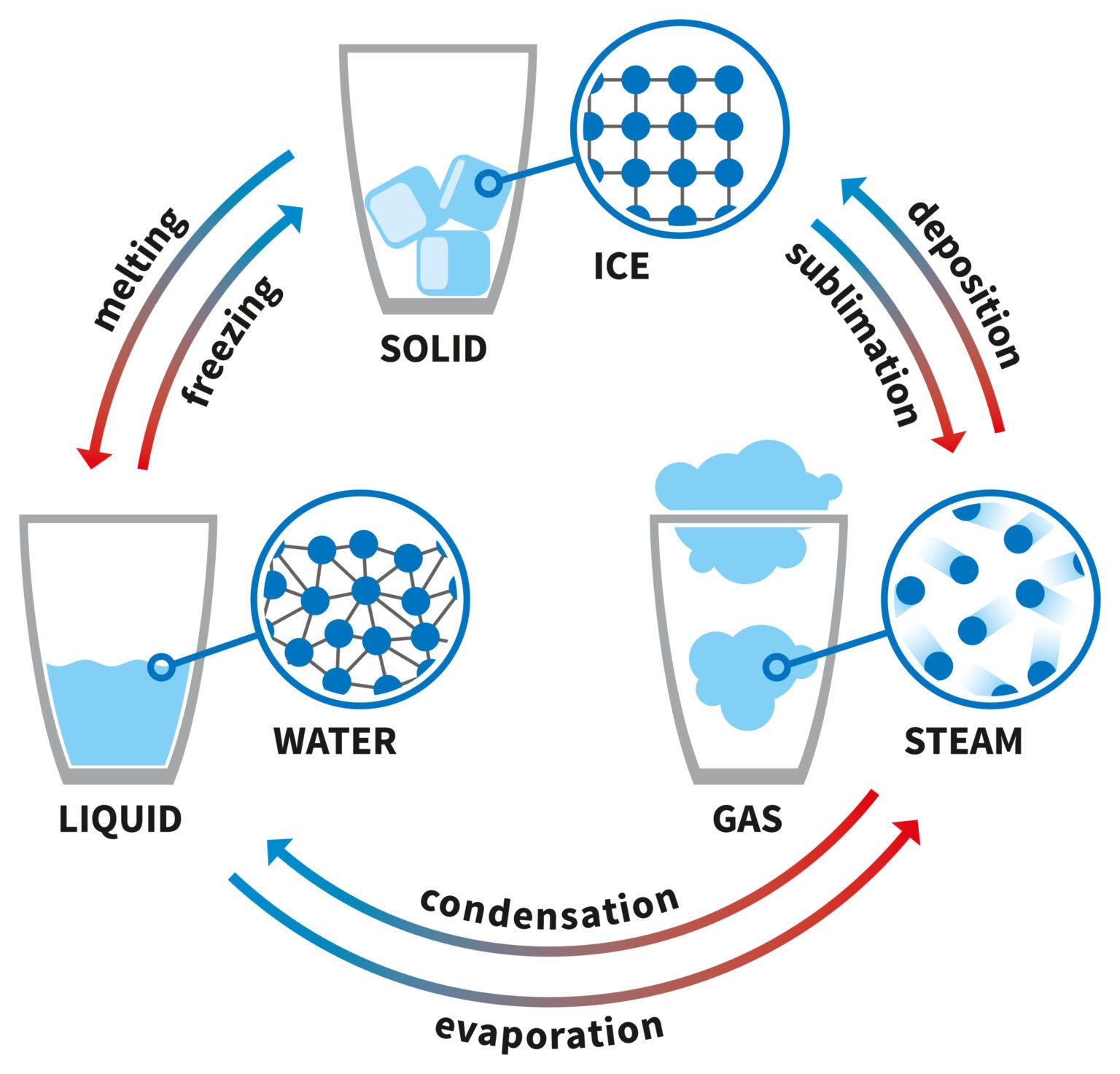
Explainer What are the different states of matter?
Do Molecules Change Shape Molecules of different shapes can adapt with their corresponding bond angles. Molecules can adapt the following shapes and bond angles: The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. This shape is dependent on the preferred spatial orientation of covalent bonds to. Shapes of molecules & ions. Then, compare the model to real. The three dimensional shape or configuration of a molecule is an important characteristic. Molecules of different shapes can adapt with their corresponding bond angles. Whether they are flat structures. According to vsepr theory, a molecule is designated by the. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. As a result, the molecule forms a regular geometric shape. How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom.
From www.snexplores.org
Explainer What are the different states of matter? Do Molecules Change Shape The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. How does molecule shape change with different numbers of bonds and electron pairs? The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Molecules of. Do Molecules Change Shape.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Do Molecules Change Shape Molecules of different shapes can adapt with their corresponding bond angles. As a result, the molecule forms a regular geometric shape. Shapes of molecules & ions. This shape is dependent on the preferred spatial orientation of covalent bonds to. According to vsepr theory, a molecule is designated by the. Molecules can adapt the following shapes and bond angles: The three. Do Molecules Change Shape.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Do Molecules Change Shape The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. This shape is dependent on the preferred spatial orientation of covalent bonds to. Molecules can adapt the following shapes and bond angles: The molecular geometry is described only by the positions of the nuclei, not. Do Molecules Change Shape.
From www.britannica.com
molecule Definition, Examples, Structures, & Facts Britannica Do Molecules Change Shape Shapes of molecules & ions. As a result, the molecule forms a regular geometric shape. How does molecule shape change with different numbers of bonds and electron pairs? According to vsepr theory, a molecule is designated by the. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Molecules of. Do Molecules Change Shape.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of Do Molecules Change Shape The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. The three dimensional shape or configuration of a molecule is an important characteristic. As a result, the molecule forms a regular geometric shape. Shapes of molecules & ions. How does molecule shape change with different. Do Molecules Change Shape.
From littletogreatscientists.com
How do Molecules React? Little to Great Scientists Do Molecules Change Shape As a result, the molecule forms a regular geometric shape. According to vsepr theory, a molecule is designated by the. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Molecules can adapt the following shapes and bond angles: The three dimensional shape or configuration of a molecule is an. Do Molecules Change Shape.
From www.pinterest.co.uk
Shapes of Molecules + Bond Angles AS chemistry Chemistry lessons Do Molecules Change Shape The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Then, compare the model to real. Shapes of molecules & ions. Molecules of different shapes can adapt with their corresponding bond angles. Find out by adding single, double or triple bonds and lone pairs to. Do Molecules Change Shape.
From chem.libretexts.org
4.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Do Molecules Change Shape Molecules can adapt the following shapes and bond angles: This shape is dependent on the preferred spatial orientation of covalent bonds to. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Then, compare the model to real. Whether they are flat structures. Find out. Do Molecules Change Shape.
From www.science-revision.co.uk
Shapes of molecules Do Molecules Change Shape This shape is dependent on the preferred spatial orientation of covalent bonds to. Molecules can adapt the following shapes and bond angles: As a result, the molecule forms a regular geometric shape. Molecules of different shapes can adapt with their corresponding bond angles. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will. Do Molecules Change Shape.
From atocwatervapor.blogspot.com
Water Phase Change Do Molecules Change Shape Molecules can adapt the following shapes and bond angles: Shapes of molecules & ions. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Whether they are flat structures. Then, compare the model to real. This shape is dependent on the preferred spatial orientation of covalent bonds to. Molecules of. Do Molecules Change Shape.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Do Molecules Change Shape According to vsepr theory, a molecule is designated by the. This shape is dependent on the preferred spatial orientation of covalent bonds to. As a result, the molecule forms a regular geometric shape. Find out by adding single, double or triple bonds and lone pairs to the central atom. Molecules of different shapes can adapt with their corresponding bond angles.. Do Molecules Change Shape.
From www.transformationtutoring.com
How To Predict Shape/Molecular Geometry Of A Molecule Do Molecules Change Shape Molecules of different shapes can adapt with their corresponding bond angles. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Shapes of molecules & ions. Whether they are flat structures. According to vsepr theory, a molecule is designated by the. Molecules can adapt the. Do Molecules Change Shape.
From chem.libretexts.org
7.2 The Gas Laws Chemistry LibreTexts Do Molecules Change Shape Then, compare the model to real. Molecules can adapt the following shapes and bond angles: According to vsepr theory, a molecule is designated by the. How does molecule shape change with different numbers of bonds and electron pairs? This shape is dependent on the preferred spatial orientation of covalent bonds to. The shapes of molecules are ultimately governed by the. Do Molecules Change Shape.
From getrevising.co.uk
States of Matter Revision Cards in IGCSE Chemistry Do Molecules Change Shape Molecules of different shapes can adapt with their corresponding bond angles. Then, compare the model to real. Whether they are flat structures. Shapes of molecules & ions. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. How does molecule shape change with different numbers of bonds and electron pairs?. Do Molecules Change Shape.
From www.youtube.com
How to Memorize Shapes of Molecules VSEPR THEORY YouTube Do Molecules Change Shape Find out by adding single, double or triple bonds and lone pairs to the central atom. Molecules of different shapes can adapt with their corresponding bond angles. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. Then, compare the model to real. Molecules can adapt the following shapes and. Do Molecules Change Shape.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations Do Molecules Change Shape The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. As a result, the molecule forms a regular geometric shape. Find out by adding single, double or triple bonds and lone pairs to the central atom. According to vsepr theory, a molecule is designated by the. Molecules can adapt the. Do Molecules Change Shape.
From www.indiapicturebudget.com
Shape of Molecules infographic diagram including linear trigonal planar Do Molecules Change Shape This shape is dependent on the preferred spatial orientation of covalent bonds to. As a result, the molecule forms a regular geometric shape. According to vsepr theory, a molecule is designated by the. The three dimensional shape or configuration of a molecule is an important characteristic. Molecules of different shapes can adapt with their corresponding bond angles. How does molecule. Do Molecules Change Shape.
From pressbooks.bccampus.ca
6.1 Writing and Balancing Chemical Equations CHEM 1114 Introduction Do Molecules Change Shape As a result, the molecule forms a regular geometric shape. According to vsepr theory, a molecule is designated by the. Whether they are flat structures. This shape is dependent on the preferred spatial orientation of covalent bonds to. Shapes of molecules & ions. How does molecule shape change with different numbers of bonds and electron pairs? Then, compare the model. Do Molecules Change Shape.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Do Molecules Change Shape The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Then, compare the model to real. Shapes of molecules & ions. Molecules of different shapes can adapt with their corresponding bond angles. The three dimensional shape or configuration of a molecule is an important characteristic.. Do Molecules Change Shape.
From www.youtube.com
Trick to learn shapes of molecules Geometry of molecules VSEPR Do Molecules Change Shape The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Molecules can adapt the following shapes and bond angles: As a result, the molecule forms a regular geometric shape. Find out by adding single, double or triple bonds and lone pairs to the central atom.. Do Molecules Change Shape.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Do Molecules Change Shape The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. According to vsepr theory, a molecule is designated by the. This shape is dependent on the preferred spatial orientation of covalent bonds to. The three dimensional shape or configuration of a molecule is an important characteristic. Molecules can adapt the. Do Molecules Change Shape.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Do Molecules Change Shape Whether they are flat structures. This shape is dependent on the preferred spatial orientation of covalent bonds to. According to vsepr theory, a molecule is designated by the. How does molecule shape change with different numbers of bonds and electron pairs? Shapes of molecules & ions. Then, compare the model to real. As a result, the molecule forms a regular. Do Molecules Change Shape.
From www.youtube.com
Shapes of Molecules Revision for ALevel Chemistry YouTube Do Molecules Change Shape Whether they are flat structures. According to vsepr theory, a molecule is designated by the. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Find out by adding single, double or triple bonds and lone pairs to the central atom. As a result, the. Do Molecules Change Shape.
From phys.org
Molecules change shape when wet Do Molecules Change Shape The three dimensional shape or configuration of a molecule is an important characteristic. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. How does. Do Molecules Change Shape.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Do Molecules Change Shape Find out by adding single, double or triple bonds and lone pairs to the central atom. This shape is dependent on the preferred spatial orientation of covalent bonds to. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. The shapes of molecules are ultimately governed by the valence electrons. Do Molecules Change Shape.
From www.science-revision.co.uk
Shapes of molecules Do Molecules Change Shape Molecules of different shapes can adapt with their corresponding bond angles. As a result, the molecule forms a regular geometric shape. How does molecule shape change with different numbers of bonds and electron pairs? Whether they are flat structures. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise. Do Molecules Change Shape.
From 2012books.lardbucket.org
Predicting the Geometry of Molecules and Polyatomic Ions Do Molecules Change Shape Whether they are flat structures. As a result, the molecule forms a regular geometric shape. According to vsepr theory, a molecule is designated by the. Molecules of different shapes can adapt with their corresponding bond angles. This shape is dependent on the preferred spatial orientation of covalent bonds to. Then, compare the model to real. The molecular geometry is described. Do Molecules Change Shape.
From enthu.com
How are Molecules Formed? EnthuZiastic Do Molecules Change Shape Find out by adding single, double or triple bonds and lone pairs to the central atom. Molecules can adapt the following shapes and bond angles: This shape is dependent on the preferred spatial orientation of covalent bonds to. According to vsepr theory, a molecule is designated by the. As a result, the molecule forms a regular geometric shape. Then, compare. Do Molecules Change Shape.
From www.acs.org
Molecule Shapes American Chemical Society Do Molecules Change Shape Shapes of molecules & ions. The three dimensional shape or configuration of a molecule is an important characteristic. As a result, the molecule forms a regular geometric shape. Molecules of different shapes can adapt with their corresponding bond angles. Find out by adding single, double or triple bonds and lone pairs to the central atom. The shapes of molecules are. Do Molecules Change Shape.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Do Molecules Change Shape This shape is dependent on the preferred spatial orientation of covalent bonds to. As a result, the molecule forms a regular geometric shape. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Find out by adding single, double or triple bonds and lone pairs. Do Molecules Change Shape.
From www.blendspace.com
Molecules, Compounds And Mixtures Lessons Blendspace Do Molecules Change Shape The three dimensional shape or configuration of a molecule is an important characteristic. Then, compare the model to real. This shape is dependent on the preferred spatial orientation of covalent bonds to. Molecules can adapt the following shapes and bond angles: Shapes of molecules & ions. Find out by adding single, double or triple bonds and lone pairs to the. Do Molecules Change Shape.
From www.nagwa.com
Question Video Representing How Intermolecular Forces Change as Do Molecules Change Shape Whether they are flat structures. As a result, the molecule forms a regular geometric shape. This shape is dependent on the preferred spatial orientation of covalent bonds to. Then, compare the model to real. The shapes of molecules are ultimately governed by the valence electrons and as a chemist you will often have to visualise molecules in your mind. Molecules. Do Molecules Change Shape.
From chemistryclinic.co.uk
Shapes of simple molecules Chemistry Do Molecules Change Shape The three dimensional shape or configuration of a molecule is an important characteristic. Then, compare the model to real. Find out by adding single, double or triple bonds and lone pairs to the central atom. How does molecule shape change with different numbers of bonds and electron pairs? As a result, the molecule forms a regular geometric shape. Shapes of. Do Molecules Change Shape.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Do Molecules Change Shape Whether they are flat structures. Find out by adding single, double or triple bonds and lone pairs to the central atom. Molecules can adapt the following shapes and bond angles: The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. This shape is dependent on the preferred spatial orientation of. Do Molecules Change Shape.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Do Molecules Change Shape Find out by adding single, double or triple bonds and lone pairs to the central atom. How does molecule shape change with different numbers of bonds and electron pairs? Molecules of different shapes can adapt with their corresponding bond angles. Then, compare the model to real. This shape is dependent on the preferred spatial orientation of covalent bonds to. The. Do Molecules Change Shape.